Exosomes derived from pioglitazone-pretreated MSCs accelerate diabetic wound healing through enhancing angiogenesis
- PMID: 34020670
- PMCID: PMC8139165
- DOI: 10.1186/s12951-021-00894-5
Exosomes derived from pioglitazone-pretreated MSCs accelerate diabetic wound healing through enhancing angiogenesis
Abstract
Background: Enhanced angiogenesis can promote diabetic wound healing. Mesenchymal stem cells (MSCs)-derived exosomes, which are cell-free therapeutics, are promising candidates for the treatment of diabetic wound healing. The present study aimed to investigate the effect of exosomes derived from MSCs pretreated with pioglitazone (PGZ-Exos) on diabetic wound healing.
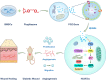
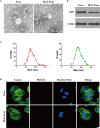
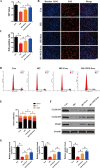
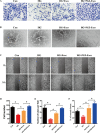
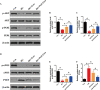
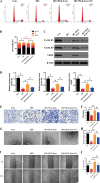
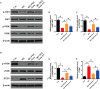
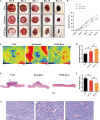
Results: We isolated PGZ-Exos from the supernatants of pioglitazone-treated BMSCs and found that PGZ-Exos significantly promote the cell viability and proliferation of Human Umbilical Vein Vascular Endothelial Cells (HUVECs) injured by high glucose (HG). PGZ-Exos enhanced the biological functions of HUVECs, including migration, tube formation, wound repair and VEGF expression in vitro. In addition, PGZ-Exos promoted the protein expression of p-AKT, p-PI3K and p-eNOS and suppressed that of PTEN. LY294002 inhibited the biological function of HUVECs through inhibition of the PI3K/AKT/eNOS pathway. In vivo modeling in diabetic rat wounds showed that pioglitazone pretreatment enhanced the therapeutic efficacy of MSCs-derived exosomes and accelerated diabetic wound healing via enhanced angiogenesis. In addition, PGZ-Exos promoted collagen deposition, ECM remodeling and VEGF and CD31 expression, indicating adequate angiogenesis in diabetic wound healing.
Conclusions: PGZ-Exos accelerated diabetic wound healing by promoting the angiogenic function of HUVECs through activation of the PI3K/AKT/eNOS pathway. This offers a promising novel cell-free therapy for treating diabetic wound healing.
Keywords: Angiogenesis; Diabetic wound; Exosomes; Mesenchymal stem cells; Pioglitazone.
Conflict of interest statement
All authors declare no conflict of interest.
Figures
Similar articles
-
Empagliflozin-Pretreated MSC-Derived Exosomes Enhance Angiogenesis and Wound Healing via PTEN/AKT/VEGF Pathway.Int J Nanomedicine. 2025 Apr 22;20:5119-5136. doi: 10.2147/IJN.S512074. eCollection 2025. Int J Nanomedicine. 2025. PMID: 40297404 Free PMC article.
-
Exosomes derived from atorvastatin-pretreated MSC accelerate diabetic wound repair by enhancing angiogenesis via AKT/eNOS pathway.Stem Cell Res Ther. 2020 Aug 12;11(1):350. doi: 10.1186/s13287-020-01824-2. Stem Cell Res Ther. 2020. PMID: 32787917 Free PMC article.
-
Serum exosomes accelerate diabetic wound healing by promoting angiogenesis and ECM formation.Cell Biol Int. 2021 Sep;45(9):1976-1985. doi: 10.1002/cbin.11627. Epub 2021 Jun 3. Cell Biol Int. 2021. PMID: 33991016
-
Exosomes from mesenchymal stem cells: Potential applications in wound healing.Life Sci. 2024 Nov 15;357:123066. doi: 10.1016/j.lfs.2024.123066. Epub 2024 Sep 19. Life Sci. 2024. PMID: 39306326 Review.
-
Exosomes: compositions, biogenesis, and mechanisms in diabetic wound healing.J Nanobiotechnology. 2024 Jul 5;22(1):398. doi: 10.1186/s12951-024-02684-1. J Nanobiotechnology. 2024. PMID: 38970103 Free PMC article. Review.
Cited by
-
How to maximize the therapeutic effect of exosomes on skin wounds in diabetes mellitus: Review and discussion.Front Endocrinol (Lausanne). 2023 Mar 27;14:1146991. doi: 10.3389/fendo.2023.1146991. eCollection 2023. Front Endocrinol (Lausanne). 2023. PMID: 37051206 Free PMC article. Review.
-
Exosomes and Their Bioengineering Strategies in the Cutaneous Wound Healing and Related Complications: Current Knowledge and Future Perspectives.Int J Biol Sci. 2023 Feb 27;19(5):1430-1454. doi: 10.7150/ijbs.80430. eCollection 2023. Int J Biol Sci. 2023. PMID: 37056923 Free PMC article. Review.
-
Mesenchymal Stem Cell Derived Exosomes Therapy in Diabetic Wound Repair.Int J Nanomedicine. 2023 May 22;18:2707-2720. doi: 10.2147/IJN.S411562. eCollection 2023. Int J Nanomedicine. 2023. PMID: 37250470 Free PMC article. Review.
-
TWEAK increases angiogenesis to promote diabetic skin wound healing by regulating Fn14/EGFR signaling.J Cosmet Dermatol. 2024 Dec;23(12):4230-4238. doi: 10.1111/jocd.16486. Epub 2024 Aug 21. J Cosmet Dermatol. 2024. PMID: 39166480 Free PMC article.
-
Precision exosome engineering for enhanced wound healing and scar revision.J Transl Med. 2025 May 23;23(1):578. doi: 10.1186/s12967-025-06578-0. J Transl Med. 2025. PMID: 40410904 Free PMC article. Review.
References
-
- Wang M, Wang C, Chen M, Xi Y, Cheng W, Mao C, Xu T, Zhang X, Lin C, Gao W, Guo Y, Lei B. Efficient angiogenesis-based diabetic wound healing/skin reconstruction through bioactive antibacterial adhesive ultraviolet shielding nanodressing with exosome release. ACS Nano. 2019;13:10279–93. doi: 10.1021/acsnano.9b03656. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

