Impact of Patient Adherence to Stool-Based Colorectal Cancer Screening and Colonoscopy Following a Positive Test on Clinical Outcomes
- PMID: 34021023
- PMCID: PMC8974412
- DOI: 10.1158/1940-6207.CAPR-21-0075
Impact of Patient Adherence to Stool-Based Colorectal Cancer Screening and Colonoscopy Following a Positive Test on Clinical Outcomes
Abstract
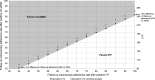
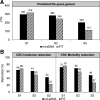
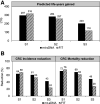
Colorectal cancer-screening models commonly assume 100% adherence, which is inconsistent with real-world experience. The influence of adherence to initial stool-based screening [fecal immunochemical test (FIT), multitarget stool DNA (mt-sDNA)] and follow-up colonoscopy (after a positive stool test) on colorectal cancer outcomes was modeled using the Colorectal Cancer and Adenoma Incidence and Mortality Microsimulation Model. Average-risk individuals without diagnosed colorectal cancer at age 40 undergoing annual FIT or triennial mt-sDNA screening from ages 50 to 75 were simulated. Primary analyses incorporated published mt-sDNA (71%) or FIT (43%) screening adherence, with follow-up colonoscopy adherence ranging from 40% to 100%. Secondary analyses simulated 100% adherence for stool-based screening and colonoscopy follow-up (S1), published adherence for stool-based screening with 100% adherence to colonoscopy follow-up (S2), and published adherence for both stool-based screening and colonoscopy follow-up after positive mt-sDNA (73%) or FIT (47%; S3). Outcomes were life-years gained (LYG) and colorectal cancer incidence and mortality reductions (per 1,000 individuals) versus no screening. Adherence to colonoscopy follow-up after FIT had to be 4%-13% higher than mt-sDNA to reach equivalent LYG. The theoretical S1 favored FIT versus mt-sDNA (LYG 316 vs. 297; colorectal cancer incidence reduction 68% vs. 64%; colorectal cancer mortality reduction 76% vs. 72%). The more realistic S2 and S3 favored mt-sDNA versus FIT (S2: LYG 284 vs. 245, colorectal cancer incidence reduction 61% vs. 50%, colorectal cancer mortality reduction 69% vs. 59%; S3: LYG 203 vs. 113, colorectal cancer incidence reduction 43% vs. 23%, colorectal cancer mortality reduction 49% vs. 27%, respectively). Incorporating realistic adherence rates for colorectal cancer screening influences modeled outcomes and should be considered when assessing comparative effectiveness. PREVENTION RELEVANCE: Adherence rates for initial colorectal cancer screening by FIT or mt-sDNA and for colonoscopy follow-up of a positive initial test influence the comparative effectiveness of these screening strategies. Using adherence rates based on published data for stool-based testing and colonoscopy follow-up yielded superior outcomes with an mt-sDNA versus FIT-screening strategy.
©2021 The Authors; Published by the American Association for Cancer Research.
Conflict of interest statement
A.M. Fendrick reports other support and has been a consultant for AbbVie, Amgen, Bayer, Centivo, Community Oncology Association, Covered California, EmblemHealth, Exact Sciences, Freedman, Health, GRAIL, Harvard University, Health and Wellness, Innovations*, Health at Scale Technologies*, HealthCorum, Hygieia, MedZed, Merck, Montana, Health Cooperative, Pair Team*, Penguin Pay, Phathom Pharmaceuticals, Risalto, Risk International, Sempre Health*, State of Minnesota, U.S., Department of Defense, Virginia Center for Health, Innovation, Wellth*, Wildflower Health, Yale-New Haven Health System, Zansors*, as well as equity Interest Research: AHRQ, Boehringer-Ingelheim, Gary and Mary West Health Policy Center, Arnold Ventures, National Pharmaceutical Council, PCORI, PhRMA, RWJ Foundation, State of Michigan/CMS Outside Position: AJMC (Co-editor), ME. D.A. Fisher reports grants and other support from Exact Sciences during the conduct of the study; as well as other support from Guardant Health outside the submitted work. L. Saoud reports personal fees from Exact Sciences during the conduct of the study. A. Ozbay reports other support from Exact Sciences during the conduct of the study; as well as other support from Exact Sciences outside the submitted work. J.J. Karlitz reports personal fees from Exact Sciences and other support from Gastro Girl/GI OnDemand outside the submitted work. P.J. Limburg reports other support from Exact Sciences during the conduct of the study; as well as other support from Exact Sciences outside the submitted work.
Figures
Similar articles
-
Impact of the Sessile Serrated Polyp Pathway on Predicted Colorectal Cancer Outcomes.Gastro Hep Adv. 2022 Feb 3;1(1):55-62. doi: 10.1016/j.gastha.2021.10.007. eCollection 2022. Gastro Hep Adv. 2022. PMID: 39129937 Free PMC article.
-
Estimating the impact of differential adherence on the comparative effectiveness of stool-based colorectal cancer screening using the CRC-AIM microsimulation model.PLoS One. 2020 Dec 29;15(12):e0244431. doi: 10.1371/journal.pone.0244431. eCollection 2020. PLoS One. 2020. PMID: 33373409 Free PMC article.
-
Cost-Effectiveness of Multitarget Stool DNA Testing vs Colonoscopy or Fecal Immunochemical Testing for Colorectal Cancer Screening in Alaska Native People.Mayo Clin Proc. 2021 May;96(5):1203-1217. doi: 10.1016/j.mayocp.2020.07.035. Epub 2021 Apr 9. Mayo Clin Proc. 2021. PMID: 33840520
-
PPV and Detection Rate of mt-sDNA Testing, FIT, and CT Colonography for Advanced Neoplasia: A Hierarchic Bayesian Meta-Analysis of the Noninvasive Colorectal Screening Tests.AJR Am J Roentgenol. 2021 Oct;217(4):817-830. doi: 10.2214/AJR.20.25416. Epub 2021 Mar 11. AJR Am J Roentgenol. 2021. PMID: 33703913
-
Influence of Colorectal Cancer Risk Factors on Predictive Value of a Positive Multitarget Stool DNA Test.J Clin Gastroenterol. 2024 May-Jun 01;58(5):471-474. doi: 10.1097/MCG.0000000000001884. Epub 2023 Jun 27. J Clin Gastroenterol. 2024. PMID: 37389965 Review.
Cited by
-
Impact of the Sessile Serrated Polyp Pathway on Predicted Colorectal Cancer Outcomes.Gastro Hep Adv. 2022 Feb 3;1(1):55-62. doi: 10.1016/j.gastha.2021.10.007. eCollection 2022. Gastro Hep Adv. 2022. PMID: 39129937 Free PMC article.
-
Cancer Screening in the United States During the Second Year of the COVID-19 Pandemic.J Clin Oncol. 2023 Sep 20;41(27):4352-4359. doi: 10.1200/JCO.22.02170. Epub 2023 Feb 23. J Clin Oncol. 2023. PMID: 36821800 Free PMC article.
-
Inappropriate Multi-Target Stool DNA Use for Colorectal Cancer Screening: Risks, Compliance, and Outcomes.Cureus. 2023 Jun 16;15(6):e40506. doi: 10.7759/cureus.40506. eCollection 2023 Jun. Cureus. 2023. PMID: 37397671 Free PMC article.
-
Clinical Validation of a Cell-Free DNA Fragmentome Assay for Augmentation of Lung Cancer Early Detection.Cancer Discov. 2024 Nov 1;14(11):2224-2242. doi: 10.1158/2159-8290.CD-24-0519. Cancer Discov. 2024. PMID: 38829053 Free PMC article.
-
Performance of different colorectal cancer screening strategies: a long-term passive follow-up population-based screening program in Beijing, China.BMC Public Health. 2023 Aug 28;23(1):1640. doi: 10.1186/s12889-023-16564-0. BMC Public Health. 2023. PMID: 37641033 Free PMC article.
References
-
- Wolf AMD, Fontham ETH, Church TR, Flowers CR, Guerra CE, LaMonte SJ, et al. Colorectal cancer screening for average-risk adults: 2018 guideline update from the American Cancer Society. CA Cancer J Clin 2018;68:250–81. - PubMed
-
- Akram A, Juang D, Bustamante R, Liu L, Earles A, Ho SB, et al. Replacing the guaiac fecal occult blood test with the fecal immunochemical test increases proportion of individuals screened in a large healthcare setting. Clin Gastroenterol Hepatol 2017;15:1265–70.e1. - PubMed
-
- Hassan C, Giorgi Rossi P, Camilloni L, Rex DK, Jimenez-Cendales B, Ferroni E, et al. Meta-analysis: adherence to colorectal cancer screening and the detection rate for advanced neoplasia, according to the type of screening test. Aliment Pharmacol Ther 2012;36:929–40. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

