Randomized phase II trial of lymphodepletion plus adoptive cell transfer of tumor-infiltrating lymphocytes, with or without dendritic cell vaccination, in patients with metastatic melanoma
- PMID: 34021033
- PMCID: PMC8144048
- DOI: 10.1136/jitc-2021-002449
Randomized phase II trial of lymphodepletion plus adoptive cell transfer of tumor-infiltrating lymphocytes, with or without dendritic cell vaccination, in patients with metastatic melanoma
Abstract
Background: The adoptive transfer of tumor-infiltrating lymphocytes (TIL) has demonstrated robust efficacy in metastatic melanoma patients. Tumor antigen-loaded dendritic cells (DCs) are believed to optimally activate antigen-specific T lymphocytes. We hypothesized that the combined transfer of TIL, containing a melanoma antigen recognized by T cells 1 (MART-1) specific population, with MART-1-pulsed DC will result in enhanced proliferation and prolonged survival of transferred MART-1 specific T cells in vivo ultimately leading to improved clinical responses.
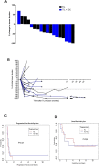
Design: We tested the combination of TIL and DC in a phase II clinical trial of patients with advanced stage IV melanoma. HLA-A0201 patients whose early TIL cultures demonstrated reactivity to MART-1 peptide were randomly assigned to receive TIL alone or TIL +DC pulsed with MART-1 peptide. The primary endpoint was to evaluate the persistence of MART-1 TIL in the two arms. Secondary endpoints were to evaluate clinical response and survival.
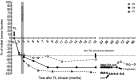
Results: Ten patients were given TIL alone while eight patients received TIL+DC vaccine. Infused MART-1 reactive CD8+ TIL were tracked in the blood over time by flow cytometry and results show good persistence in both arms, with no difference in the persistence of MART-1 between the two arms. The objective response rate was 30% (3/10) in the TIL arm and 50% (4/8) in the TIL+DC arm. All treatments were well tolerated.
Conclusions: The combination of TIL +DC showed no difference in the persistence of MART-1 TIL compared with TIL therapy alone. Although more patients showed a clinical response to TIL+DC therapy, this study was not powered to resolve differences between groups.
Trial registration number: NCT00338377.
Keywords: adaptive immunity; dendritic cells; lymphocytes; melanoma; tumor-infiltrating; vaccination.
© Author(s) (or their employer(s)) 2021. Re-use permitted under CC BY. Published by BMJ.
Conflict of interest statement
Competing interests: CLH is on the SAB for Briacell. PH consulting agreement with Immatics, Dragonfly, Sanofi, GSK. CB is on the SAB of Myst therapeutics and received research funding from Iovance biotherapeutics.
Figures



Similar articles
-
Adoptive transfer of MART-1 T-cell receptor transgenic lymphocytes and dendritic cell vaccination in patients with metastatic melanoma.Clin Cancer Res. 2014 May 1;20(9):2457-65. doi: 10.1158/1078-0432.CCR-13-3017. Epub 2014 Mar 14. Clin Cancer Res. 2014. PMID: 24634374 Free PMC article. Clinical Trial.
-
A phase I clinical trial combining dendritic cell vaccination with adoptive T cell transfer in patients with stage IV melanoma.Cancer Immunol Immunother. 2014 Oct;63(10):1061-71. doi: 10.1007/s00262-014-1575-2. Epub 2014 Jul 4. Cancer Immunol Immunother. 2014. PMID: 24993563 Free PMC article. Clinical Trial.
-
Infusion of Melan-A/Mart-1 specific tumor-infiltrating lymphocytes enhanced relapse-free survival of melanoma patients.Cancer Immunol Immunother. 2007 Apr;56(4):515-26. doi: 10.1007/s00262-006-0204-0. Epub 2006 Jul 28. Cancer Immunol Immunother. 2007. PMID: 16874485 Free PMC article. Clinical Trial.
-
White paper on adoptive cell therapy for cancer with tumor-infiltrating lymphocytes: a report of the CTEP subcommittee on adoptive cell therapy.Clin Cancer Res. 2011 Apr 1;17(7):1664-73. doi: 10.1158/1078-0432.CCR-10-2272. Epub 2011 Feb 15. Clin Cancer Res. 2011. PMID: 21325070 Review.
-
Focus on adoptive T cell transfer trials in melanoma.Clin Dev Immunol. 2010;2010:260267. doi: 10.1155/2010/260267. Epub 2010 Dec 26. Clin Dev Immunol. 2010. PMID: 21234353 Free PMC article. Review.
Cited by
-
Immune killer cells treatment for previously treated stage IV NSCLC patients.Sci Rep. 2024 Aug 21;14(1):19374. doi: 10.1038/s41598-024-69587-x. Sci Rep. 2024. PMID: 39169058 Free PMC article. Clinical Trial.
-
Prognostic significance of peripheral and tumor-infiltrating lymphocytes in newly diagnosed stage III/IV non-small-cell lung cancer.Front Med (Lausanne). 2024 May 22;11:1349178. doi: 10.3389/fmed.2024.1349178. eCollection 2024. Front Med (Lausanne). 2024. PMID: 38841570 Free PMC article.
-
Lifileucel: FDA-approved T-cell therapy for melanoma.Oncologist. 2024 Aug 5;29(8):648-650. doi: 10.1093/oncolo/oyae136. Oncologist. 2024. PMID: 39012213 Free PMC article.
-
The Role of the Tumor Microenvironment (TME) in Advancing Cancer Therapies: Immune System Interactions, Tumor-Infiltrating Lymphocytes (TILs), and the Role of Exosomes and Inflammasomes.Int J Mol Sci. 2025 Mar 18;26(6):2716. doi: 10.3390/ijms26062716. Int J Mol Sci. 2025. PMID: 40141358 Free PMC article. Review.
-
Efficacy of treatment with tumor-infiltrating lymphocytes as adoptive cell therapy: an integrative review.Einstein (Sao Paulo). 2024 Dec 16;22:eRW0935. doi: 10.31744/einstein_journal/2024RW0935. eCollection 2024. Einstein (Sao Paulo). 2024. PMID: 39699412 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
