Protein Phosphatase 2B Dual Function Facilitates Synaptic Integrity and Motor Learning
- PMID: 34021041
- PMCID: PMC8244972
- DOI: 10.1523/JNEUROSCI.1741-20.2021
Protein Phosphatase 2B Dual Function Facilitates Synaptic Integrity and Motor Learning
Abstract
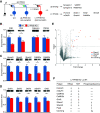
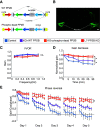
Protein phosphatase 2B (PP2B) is critical for synaptic plasticity and learning, but the molecular mechanisms involved remain unclear. Here we identified different types of proteins that interact with PP2B, including various structural proteins of the postsynaptic densities (PSDs) of Purkinje cells (PCs) in mice. Deleting PP2B reduced expression of PSD proteins and the relative thickness of PSD at the parallel fiber to PC synapses, whereas reexpression of inactive PP2B partly restored the impaired distribution of nanoclusters of PSD proteins, together indicating a structural role of PP2B. In contrast, lateral mobility of surface glutamate receptors solely depended on PP2B phosphatase activity. Finally, the level of motor learning covaried with both the enzymatic and nonenzymatic functions of PP2B. Thus, PP2B controls synaptic function and learning both through its action as a phosphatase and as a structural protein that facilitates synapse integrity.SIGNIFICANCE STATEMENT Phosphatases are generally considered to serve their critical role in learning and memory through their enzymatic operations. Here, we show that protein phosphatase 2B (PP2B) interacts with structural proteins at the synapses of cerebellar Purkinje cells. Differentially manipulating the enzymatic and structural domains of PP2B leads to different phenotypes in cerebellar learning. We propose that PP2B is crucial for cerebellar learning via two complementary actions, an enzymatic and a structural operation.
Keywords: Purkinje cells; cerebellar learning; protein phosphatase 2B.
Copyright © 2021 Lin et al.
Figures





Similar articles
-
PP2B-Dependent Cerebellar Plasticity Sets the Amplitude of the Vestibulo-ocular Reflex during Juvenile Development.J Neurosci. 2024 Apr 24;44(17):e1211232024. doi: 10.1523/JNEUROSCI.1211-23.2024. J Neurosci. 2024. PMID: 38527808 Free PMC article.
-
Purkinje cell-specific knockout of the protein phosphatase PP2B impairs potentiation and cerebellar motor learning.Neuron. 2010 Aug 26;67(4):618-28. doi: 10.1016/j.neuron.2010.07.009. Neuron. 2010. PMID: 20797538 Free PMC article.
-
Binding of Filamentous Actin to CaMKII as Potential Regulation Mechanism of Bidirectional Synaptic Plasticity by β CaMKII in Cerebellar Purkinje Cells.Sci Rep. 2020 Jun 2;10(1):9019. doi: 10.1038/s41598-020-65870-9. Sci Rep. 2020. PMID: 32488204 Free PMC article.
-
The role of calcium in synaptic plasticity and motor learning in the cerebellar cortex.Neurosci Biobehav Rev. 2012 Apr;36(4):1153-62. doi: 10.1016/j.neubiorev.2012.01.005. Epub 2012 Jan 28. Neurosci Biobehav Rev. 2012. PMID: 22305995 Review.
-
Synaptic memories upside down: bidirectional plasticity at cerebellar parallel fiber-Purkinje cell synapses.Neuron. 2006 Oct 19;52(2):227-38. doi: 10.1016/j.neuron.2006.09.032. Neuron. 2006. PMID: 17046686 Review.
Cited by
-
PP2B-Dependent Cerebellar Plasticity Sets the Amplitude of the Vestibulo-ocular Reflex during Juvenile Development.J Neurosci. 2024 Apr 24;44(17):e1211232024. doi: 10.1523/JNEUROSCI.1211-23.2024. J Neurosci. 2024. PMID: 38527808 Free PMC article.
-
Phosphatase-independent activity of smooth-muscle calcineurin orchestrates a gene expression program leading to hypertension.PLoS Biol. 2025 May 14;23(5):e3003163. doi: 10.1371/journal.pbio.3003163. eCollection 2025 May. PLoS Biol. 2025. PMID: 40367288 Free PMC article.
References
-
- Albus J (1971) A theory of cerebellar function. Math Biosci 10:25–61. 10.1016/0025-5564(71)90051-4 - DOI
-
- Böhm G, Prefot P, Jung S, Selzer S, Mitra V, Britton D, Kuhn K, Pike I, Thompson AH (2015) Low-pH solid-phase amino labeling of complex peptide digests with TMTs improves peptide identification rates for multiplexed global phosphopeptide analysis. J Proteome Res 14:2500–2510. 10.1021/acs.jproteome.5b00072 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
