Inoculum effect of antimicrobial peptides
- PMID: 34021080
- PMCID: PMC8166072
- DOI: 10.1073/pnas.2014364118
Inoculum effect of antimicrobial peptides
Abstract
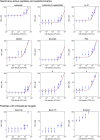
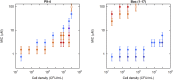
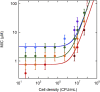
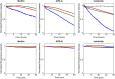
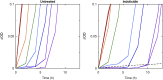
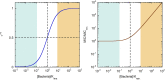
The activity of many antibiotics depends on the initial density of cells used in bacterial growth inhibition assays. This phenomenon, termed the inoculum effect, can have important consequences for the therapeutic efficacy of the drugs, because bacterial loads vary by several orders of magnitude in clinically relevant infections. Antimicrobial peptides are a promising class of molecules in the fight against drug-resistant bacteria because they act mainly by perturbing the cell membranes rather than by inhibiting intracellular targets. Here, we report a systematic characterization of the inoculum effect for this class of antibacterial compounds. Minimum inhibitory concentration values were measured for 13 peptides (including all-D enantiomers) and peptidomimetics, covering more than seven orders of magnitude in inoculated cell density. In most cases, the inoculum effect was significant for cell densities above the standard inoculum of 5 × 105 cells/mL, while for lower densities the active concentrations remained essentially constant, with values in the micromolar range. In the case of membrane-active peptides, these data can be rationalized by considering a simple model, taking into account peptide-cell association, and hypothesizing that a threshold number of cell-bound peptide molecules is required in order to cause bacterial killing. The observed effect questions the clinical utility of activity and selectivity determinations performed at a fixed, standardized cell density. A routine evaluation of the dependence of the activity of antimicrobial peptides and peptidomimetics on the inoculum should be considered.
Keywords: antimicrobial activity; antimicrobial peptides; inoculum effect.
Conflict of interest statement
The authors declare no competing interest.
Figures
References
-
- Andrews J. M., Determination of minimum inhibitory concentrations. J. Antimicrob. Chemother. 48 (suppl. 1), 5–16 (2001). - PubMed
-
- Wiegand I., Hilpert K., Hancock R. E., Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nat. Protoc. 3, 163–175 (2008). - PubMed
-
- Patel J. B., et al. ., M07-A10: Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically, Approved Standard (Clinical and Laboratory Standards Institute, Wayne, PA, ed. 10, 2015).
-
- Weinstein M. P., et al. ., Performance Standards for Antimicrobial Susceptibility Testing (Clinical and Laboratory Standards Institute, Wayne, PA, ed. 29, 2019).
-
- European Committee for Antimicrobial Susceptibility Testing (EUCAST) of the European Society of Clinical Microbiology and Infectious Diseases (ESCMID) , Determination of minimum inhibitory concentrations (MICs) of antibacterial agents by broth dilution. Clin. Microbiol. Infect. 9, ix–xv (2003). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

