Elementary mechanisms of calmodulin regulation of NaV1.5 producing divergent arrhythmogenic phenotypes
- PMID: 34021086
- PMCID: PMC8166197
- DOI: 10.1073/pnas.2025085118
Elementary mechanisms of calmodulin regulation of NaV1.5 producing divergent arrhythmogenic phenotypes
Abstract
In cardiomyocytes, NaV1.5 channels mediate initiation and fast propagation of action potentials. The Ca2+-binding protein calmodulin (CaM) serves as a de facto subunit of NaV1.5. Genetic studies and atomic structures suggest that this interaction is pathophysiologically critical, as human mutations within the NaV1.5 carboxy-terminus that disrupt CaM binding are linked to distinct forms of life-threatening arrhythmias, including long QT syndrome 3, a "gain-of-function" defect, and Brugada syndrome, a "loss-of-function" phenotype. Yet, how a common disruption in CaM binding engenders divergent effects on NaV1.5 gating is not fully understood, though vital for elucidating arrhythmogenic mechanisms and for developing new therapies. Here, using extensive single-channel analysis, we find that the disruption of Ca2+-free CaM preassociation with NaV1.5 exerts two disparate effects: 1) a decrease in the peak open probability and 2) an increase in persistent NaV openings. Mechanistically, these effects arise from a CaM-dependent switch in the NaV inactivation mechanism. Specifically, CaM-bound channels preferentially inactivate from the open state, while those devoid of CaM exhibit enhanced closed-state inactivation. Further enriching this scheme, for certain mutant NaV1.5, local Ca2+ fluctuations elicit a rapid recruitment of CaM that reverses the increase in persistent Na current, a factor that may promote beat-to-beat variability in late Na current. In all, these findings identify the elementary mechanism of CaM regulation of NaV1.5 and, in so doing, unravel a noncanonical role for CaM in tuning ion channel gating. Furthermore, our results furnish an in-depth molecular framework for understanding complex arrhythmogenic phenotypes of NaV1.5 channelopathies.
Keywords: Brugada syndrome; Nav1.5; calmodulin; ion channels; long QT syndrome.
Conflict of interest statement
The authors declare no competing interest .
Figures

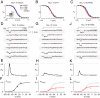

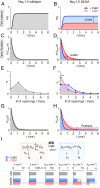
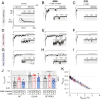
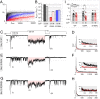

References
-
- Hille B., Ionic Channels of Excitable Membranes (Sinauer Associates, Sunderland, MA, 1984), pp. 226–248.
-
- Catterall W. A., Lenaeus M. J., Gamal El-Din T. M., Structure and pharmacology of voltage-gated sodium and calcium channels. Annu. Rev. Pharmacol. Toxicol. 60, 133–154 (2020). - PubMed
-
- Zimmer T., Surber R., SCN5A channelopathies–An update on mutations and mechanisms. Prog. Biophys. Mol. Biol. 98, 120–136 (2008). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

