Qki activates Srebp2-mediated cholesterol biosynthesis for maintenance of eye lens transparency
- PMID: 34021134
- PMCID: PMC8139980
- DOI: 10.1038/s41467-021-22782-0
Qki activates Srebp2-mediated cholesterol biosynthesis for maintenance of eye lens transparency
Abstract
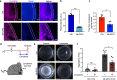
Defective cholesterol biosynthesis in eye lens cells is often associated with cataracts; however, how genes involved in cholesterol biosynthesis are regulated in lens cells remains unclear. Here, we show that Quaking (Qki) is required for the transcriptional activation of genes involved in cholesterol biosynthesis in the eye lens. At the transcriptome level, lens-specific Qki-deficient mice present downregulation of genes associated with the cholesterol biosynthesis pathway, resulting in a significant reduction of total cholesterol level in the eye lens. Mice with Qki depletion in lens epithelium display progressive accumulation of protein aggregates, eventually leading to cataracts. Notably, these defects are attenuated by topical sterol administration. Mechanistically, we demonstrate that Qki enhances cholesterol biosynthesis by recruiting Srebp2 and Pol II in the promoter regions of cholesterol biosynthesis genes. Supporting its function as a transcription co-activator, we show that Qki directly interacts with single-stranded DNA. In conclusion, we propose that Qki-Srebp2-mediated cholesterol biosynthesis is essential for maintaining the cholesterol level that protects lens from cataract development.
Conflict of interest statement
The authors declare no competing interests.
Figures






Similar articles
-
Qki regulates myelinogenesis through Srebp2-dependent cholesterol biosynthesis.Elife. 2021 May 4;10:e60467. doi: 10.7554/eLife.60467. Elife. 2021. PMID: 33942715 Free PMC article.
-
Ring finger protein 5 activates sterol regulatory element-binding protein 2 (SREBP2) to promote cholesterol biosynthesis via inducing polyubiquitination of SREBP chaperone SCAP.J Biol Chem. 2020 Mar 20;295(12):3918-3928. doi: 10.1074/jbc.RA119.011849. Epub 2020 Feb 13. J Biol Chem. 2020. PMID: 32054686 Free PMC article.
-
Downregulation of cholesterol biosynthesis genes in the forebrain of ERCC1-deficient mice.Neurobiol Dis. 2012 Mar;45(3):1136-44. doi: 10.1016/j.nbd.2011.12.036. Epub 2011 Dec 29. Neurobiol Dis. 2012. PMID: 22245387 Free PMC article.
-
Cholesterol biosynthesis supports the growth of hepatocarcinoma lesions depleted of fatty acid synthase in mice and humans.Gut. 2020 Jan;69(1):177-186. doi: 10.1136/gutjnl-2018-317581. Epub 2019 Apr 6. Gut. 2020. PMID: 30954949 Free PMC article.
-
A spontaneous mutation in Srebf2 leads to cataracts and persistent skin wounds in the lens opacity 13 (lop13) mouse.Mamm Genome. 2011 Dec;22(11-12):661-73. doi: 10.1007/s00335-011-9354-2. Epub 2011 Aug 21. Mamm Genome. 2011. PMID: 21858719 Free PMC article.
Cited by
-
Myelin lipid metabolism and its role in myelination and myelin maintenance.Innovation (Camb). 2022 Dec 7;4(1):100360. doi: 10.1016/j.xinn.2022.100360. eCollection 2023 Jan 30. Innovation (Camb). 2022. PMID: 36588745 Free PMC article. Review.
-
Immune landscape of a genetically engineered murine model of glioma compared with human glioma.JCI Insight. 2022 Jun 22;7(12):e148990. doi: 10.1172/jci.insight.148990. JCI Insight. 2022. PMID: 35653194 Free PMC article.
-
Cholesterol Content Regulates the Interaction of αA-, αB-, and α-Crystallin with the Model of Human Lens-Lipid Membranes.Int J Mol Sci. 2024 Feb 5;25(3):1923. doi: 10.3390/ijms25031923. Int J Mol Sci. 2024. PMID: 38339200 Free PMC article.
-
Lanosterol Synthase Prevents EMT During Lens Epithelial Fibrosis Via Regulating SREBP1.Invest Ophthalmol Vis Sci. 2023 Dec 1;64(15):12. doi: 10.1167/iovs.64.15.12. Invest Ophthalmol Vis Sci. 2023. PMID: 38079167 Free PMC article.
-
RNA-binding proteins and post-transcriptional regulation in lens biology and cataract: Mediating spatiotemporal expression of key factors that control the cell cycle, transcription, cytoskeleton and transparency.Exp Eye Res. 2022 Jan;214:108889. doi: 10.1016/j.exer.2021.108889. Epub 2021 Dec 11. Exp Eye Res. 2022. PMID: 34906599 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

