Trabecular bone organoids: a micron-scale 'humanised' prototype designed to study the effects of microgravity and degeneration
- PMID: 34021163
- PMCID: PMC8140135
- DOI: 10.1038/s41526-021-00146-8
Trabecular bone organoids: a micron-scale 'humanised' prototype designed to study the effects of microgravity and degeneration
Abstract
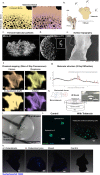
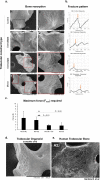
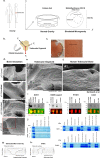
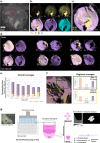
Bone is a highly responsive organ, which continuously adapts to the environment it is subjected to in order to withstand metabolic demands. These events are difficult to study in this particular tissue in vivo, due to its rigid, mineralised structure and inaccessibility of the cellular component located within. This manuscript presents the development of a micron-scale bone organoid prototype, a concept that can allow the study of bone processes at the cell-tissue interface. The model is constructed with a combination of primary female osteoblastic and osteoclastic cells, seeded onto femoral head micro-trabeculae, where they recapitulate relevant phenotypes and functions. Subsequently, constructs are inserted into a simulated microgravity bioreactor (NASA-Synthecon) to model a pathological state of reduced mechanical stimulation. In these constructs, we detected osteoclastic bone resorption sites, which were different in morphology in the simulated microgravity group compared to static controls. Once encapsulated in human fibrin and exposed to analogue microgravity for 5 days, masses of bone can be observed being lost from the initial structure, allowing to simulate the bone loss process further. Constructs can function as multicellular, organotypic units. Large osteocytic projections and tubular structures develop from the initial construct into the matrix at the millimetre scale. Micron-level fragments from the initial bone structure are detected travelling along these tubules and carried to sites distant from the native structure, where new matrix formation is initiated. We believe this model allows the study of fine-level physiological processes, which can shed light into pathological bone loss and imbalances in bone remodelling.
Conflict of interest statement
The authors declare no competing interests.
Figures




References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

