Point-of-care lung ultrasound in COVID-19 patients: inter- and intra-observer agreement in a prospective observational study
- PMID: 34021219
- PMCID: PMC8139973
- DOI: 10.1038/s41598-021-90153-2
Point-of-care lung ultrasound in COVID-19 patients: inter- and intra-observer agreement in a prospective observational study
Abstract
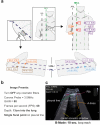
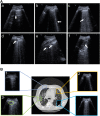
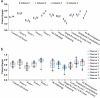
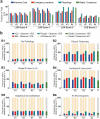
With an urgent need for bedside imaging of coronavirus disease 2019 (COVID-19), this study's main goal was to assess inter- and intraobserver agreement in lung ultrasound (LUS) of COVID-19 patients. In this single-center study we prospectively acquired and evaluated 100 recorded ten-second cine-loops in confirmed COVID-19 intensive care unit (ICU) patients. All loops were rated by ten observers with different subspeciality backgrounds for four times by each observer (400 loops overall) in a random sequence using a web-based rating tool. We analyzed inter- and intraobserver variability for specific pathologies and a semiquantitative LUS score. Interobserver agreement for both, identification of specific pathologies and assignment of LUS scores was fair to moderate (e.g., LUS score 1 Fleiss' κ = 0.27; subpleural consolidations Fleiss' κ = 0.59). Intraobserver agreement was mostly moderate to substantial with generally higher agreement for more distinct findings (e.g., lowest LUS score 0 vs. highest LUS score 3 (median Fleiss' κ = 0.71 vs. 0.79) or air bronchograms (median Fleiss' κ = 0.72)). Intraobserver consistency was relatively low for intermediate LUS scores (e.g. LUS Score 1 median Fleiss' κ = 0.52). We therefore conclude that more distinct LUS findings (e.g., air bronchograms, subpleural consolidations) may be more suitable for disease monitoring, especially with more than one investigator and that training material used for LUS in point-of-care ultrasound (POCUS) should pay refined attention to areas such as B-line quantification and differentiation of intermediate LUS scores.
Conflict of interest statement
The authors declare no competing interests.
Figures
References
-
- Rodriguez-Morales AJ, Cardona-Ospina JA, Gutiérrez-Ocampo E, Villamizar-Peña R, Holguin-Rivera Y, Escalera-Antezana JP, et al. Clinical, laboratory and imaging features of COVID-19: A systematic review and meta-analysis. Travel Med. Infect. Dis. 2020;34:101623. doi: 10.1016/j.tmaid.2020.101623. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

