International consensus on initial screening and follow-up of asymptomatic SDHx mutation carriers
- PMID: 34021277
- PMCID: PMC8205850
- DOI: 10.1038/s41574-021-00492-3
International consensus on initial screening and follow-up of asymptomatic SDHx mutation carriers
Abstract
Approximately 20% of patients diagnosed with a phaeochromocytoma or paraganglioma carry a germline mutation in one of the succinate dehydrogenase (SDHx) genes (SDHA, SDHB, SDHC and SDHD), which encode the four subunits of the SDH enzyme. When a pathogenic SDHx mutation is identified in an affected patient, genetic counselling is proposed for first-degree relatives. Optimal initial evaluation and follow-up of people who are asymptomatic but might carry SDHx mutations have not yet been agreed. Thus, we established an international consensus algorithm of clinical, biochemical and imaging screening at diagnosis and during surveillance for both adults and children. An international panel of 29 experts from 12 countries was assembled, and the Delphi method was used to reach a consensus on 41 statements. This Consensus Statement covers a range of topics, including age of first genetic testing, appropriate biochemical and imaging tests for initial tumour screening and follow-up, screening for rare SDHx-related tumours and management of elderly people who have an SDHx mutation. This Consensus Statement focuses on the management of asymptomatic SDHx mutation carriers and provides clinicians with much-needed guidance. The standardization of practice will enable prospective studies in the near future.
Conflict of interest statement
The authors declare no competing interests.
Figures
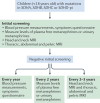
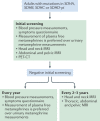
Comment in
-
Asymptomatic carriers of mutations in succinate dehydrogenase (SDHx) genes. In search of consensus for follow-up.Endocrinol Diabetes Nutr (Engl Ed). 2022 Mar;69(3):157-159. doi: 10.1016/j.endien.2022.01.008. Epub 2022 Mar 4. Endocrinol Diabetes Nutr (Engl Ed). 2022. PMID: 35353683 No abstract available.
References
-
- Ben Aim L, et al. Targeted next-generation sequencing detects rare genetic events in pheochromocytoma and paraganglioma. J. Med. Genet. 2019;56:513–520. - PubMed
-
- Lenders JWM, et al. Pheochromocytoma and paraganglioma: an endocrine society clinical practice guideline. J. Clin. Endocrinol. Metab. 2014;99:1915–1942. - PubMed
-
- Buffet A, et al. Positive impact of genetic test on the management and outcome of patients with paraganglioma and/or pheochromocytoma. J. Clin. Endocrinol. Metab. 2019;104:1109–1118. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

