"Good practices" in pediatric clinical care for disorders/differences of sex development
- PMID: 34021489
- PMCID: PMC8325784
- DOI: 10.1007/s12020-021-02748-4
"Good practices" in pediatric clinical care for disorders/differences of sex development
Abstract
Purpose: To define, benchmark, and publicize elements of quality care (i.e., "good practices") for pediatric patients with disorders/differences of sex development (DSD).
Methods: Principles of quality care were identified by literature review; consensus exists for 11 good practices and adherence was evaluated through online survey of 21 North American clinical sites.
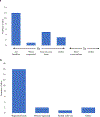
Results: Strong uptake was observed for many practices, particularly specialty participation (n ≥ 17 of 21 sites for most core specialties); point of contact (n = 18); expertise in gender dysphoria/dissatisfaction (n = 20); and DSD-specific continuing medical education (n = 18). Greater variability was apparent for frequency of peer support referrals (n = 12 universally practiced); standardized questionnaires for routine assessment of psychosocial adaptation (n = 13) and gender development (n = 10); consistently clarifying patient/family values in decision-making (n = 15); genital exam protocols that exclude trainee education as primary reason (n = 15); and internal patient-tracking efforts (n = 5-10 of 20 sites).
Conclusion: This study employed a novel approach to designate DSD good practices and identified areas of consistency and variation in these DSD clinical practices. Good practice benchmarking facilitates quality assessment within and across sites, promotes continuous improvement, and empowers stakeholders in locating and delivering high quality care.
Keywords: Benchmarking; Disorders of sex development; Guideline; Quality improvement.
© 2021. The Author(s), under exclusive licence to Springer Science+Business Media, LLC, part of Springer Nature.
Conflict of interest statement
Declarations
Figures

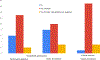
References
-
- Lee PA, Houk CP, Ahmed SF, Hughes IA, International Consensus Conference on Intersex organized by the Lawson Wilkins Pediatric Endocrine Society and the European Society for Paediatric Endocrinology: Consensus statement on management of intersex disorders. Pediatrics 118, e488–500 (2006). 10.1542/peds.2006-0738 - DOI - PubMed
-
- Institute of Medicine: Clinical practice guidelines we can trust. The National Academies Press, Washington, DC: (2011). 10.17226/13058 - DOI
-
- Speiser PW, Arlt W, Auchus RJ, Baskin LS, Conway GS, Merke DP, Meyer-Bahlburg H, Miller WL, Murad MH, Oberfield SE, White PC: Congenital adrenal hyperplasia due to steroid 21-hydroxylase deficiency: An Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab 103, 4043–4088 (2018). 10.1210/jc.2018-01865 - DOI - PMC - PubMed
-
- Gravholt CH, Andersen NH, Conway GS, Dekkers OM, Geffner ME, Klein KO, Lin AE, Mauras N, Quigley CA, Rubin K, Sandberg DE, Sas T, Silberbach M, Söderström-Anttila V, Stochholm K, van Alfen-van derVelden JA, Woelfle J, Backeljauw PF, International Turner Syndrome Consensus Group: Clinical practice guidelines for the care of girls and women with Turner syndrome: proceedings from the 2016 Cincinnati International Turner Syndrome Meeting. Eur J Endocrinol 177, G1–G70 (2017). 10.1530/EJE-17-0430 - DOI - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

