Melanosome Biogenesis in the Pigmentation of Mammalian Skin
- PMID: 34021746
- PMCID: PMC8516112
- DOI: 10.1093/icb/icab078
Melanosome Biogenesis in the Pigmentation of Mammalian Skin
Abstract
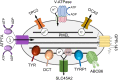
Melanins, the main pigments of the skin and hair in mammals, are synthesized within membrane-bound organelles of melanocytes called melanosomes. Melanosome structure and function are determined by a cohort of resident transmembrane proteins, many of which are expressed only in pigment cells and localize specifically to melanosomes. Defects in the genes that encode melanosome-specific proteins or components of the machinery required for their transport in and out of melanosomes underlie various forms of ocular or oculocutaneous albinism, characterized by hypopigmentation of the hair, skin, and eyes and by visual impairment. We review major components of melanosomes, including the enzymes that catalyze steps in melanin synthesis from tyrosine precursors, solute transporters that allow these enzymes to function, and structural proteins that underlie melanosome shape and melanin deposition. We then review the molecular mechanisms by which these components are biosynthetically delivered to newly forming melanosomes-many of which are shared by other cell types that generate cell type-specific lysosome-related organelles. We also highlight unanswered questions that need to be addressed by future investigation.
© The Author(s) 2021. Published by Oxford University Press on behalf of the Society for Integrative and Comparative Biology. All rights reserved. For permissions please email: journals.permissions@oup.com.
Figures


References
-
- Altimimi HF, Schnetkamp PP.. 2007. Na+/Ca2+-K+ exchangers (NCKX): functional properties and physiological roles. Channels (Austin) 1:62–9. - PubMed
-
- Ancans J, Tobin DJ, Hoogduijn MJ, Smit NP, Wakamatsu K, Thody AJ.. 2001. Melanosomal pH controls rate of melanogenesis, eumelanin/phaeomelanin ratio and melanosome maturation in melanocytes and melanoma cells. Exp Cell Res 268:26–35. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 AR071382/AR/NIAMS NIH HHS/United States
- R01 AR076241/AR/NIAMS NIH HHS/United States
- R01 EY015625/EY/NEI NIH HHS/United States
- R01 EY015625/NH/NIH HHS/United States
- R01 AR076241/EY/NEI NIH HHS/United States
- National Institute for Arthritis, Musculoskeletal and Skin Diseases
- DEQU201903007827/Fondation pour la Recherche Médicale
- ARCPJA22020060002267/Fondation ARC pour la Recherche sur le Cancer
- ANR-17-CE11-0029-03/Agence Nationale de la Recherche
- ANR-11-LABX-0038/LabEx Cell(n)Scale
- L'Oréal Research and Development (to G.R.)
- Institut Curie (to G.R.), l'Institut National de la Santé et de la Recherche Médicale (INSERM, to C.D.), and the Centre National de la Recherche Scientifique (CNRS, to G.R.)
LinkOut - more resources
Full Text Sources
Other Literature Sources

