Mouthrinses against SARS-CoV-2: anti-inflammatory effectivity and a clinical pilot study
- PMID: 34021807
- PMCID: PMC8140561
- DOI: 10.1007/s00405-021-06873-8
Mouthrinses against SARS-CoV-2: anti-inflammatory effectivity and a clinical pilot study
Abstract
Purpose: The scope of this research endeavor was the determination of the applicability of over the counter mouthwash solutions in reducing the viral load in the saliva of COVID-19 patients and hence decreasing their infectivity. Beyond that, new experimental mouthwashes were investigated in terms of a possible positive immune modulation, which might offer an additional opportunity for a positive pharmaceutical effect.
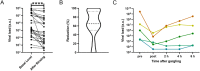
Methods: The effectivity of the mouth washing solution was determined on 34 hospitalized COVID-19 patients by measuring the viral load by RT-qPCR in pharyngeal swabs, which were taken before and after rinsing. The inflammatory modulation thru the experimental solutions was assayed in an in vitro model of virus infected nasopharyngeal epithelium cells.
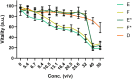
Results: The clinical pilot study demonstrated that the mouth rinsing solution was able to reduce the viral load by about 90% in the saliva of most patients. This reduction was determined to persist for about 6 h. In the experimental solutions, the ingredients dexpanthenol and zinc were able to reduce the expression of proinflammatory cytokines in the cell culture model, while the antiviral response was not altered significantly.
Conclusion: We recommend the application of mouth wash solutions to COVID-19 patients, since our results indicate a reduction in infectivity and might govern the protection of health care professionals. Further improvement to the over the counter formulation can be made by utilizing zinc and dexpanthenol, as they which might be beneficial for the patients' health.
Keywords: Anti-inflammatory; Covid-19; Dexpanthenol; Mouth wash; Zinc.
© 2021. The Author(s).
Conflict of interest statement
The authors disclose no conflicts of interest.
Figures
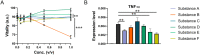
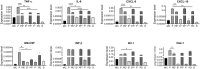
References
-
- Blanco-melo D, Nilsson-payant BE, Liu WC, Uhl S, Hoagland D, Moller R, Jordan TX, Oishi K, Panis M, Sachs D, Wang TT, Schwartz RE, Lim JK, Albrecht RA, Tenoever BR. Imbalanced host response to SARS-CoV-2 drives development of COVID-19. Cell. 2020;181:1036–1045. doi: 10.1016/j.cell.2020.04.026. - DOI - PMC - PubMed
-
- Choudhury MIM, Shabnam N, Tazin Ahsan M. Effect of 1% povidone iodine mouthwash/gargle, nasal and eye drop in COVID-19 patient. Biores Commun. 2021;7:919–923. doi: 10.3329/brc.v7i1.54245. - DOI
-
- Chu H, Chan JF, Wang Y, Yuen TT, Chai Y, Hou Y, Shuai H, Yang D, Hu B, Huang X, Zhang X, Cai JP, Zhou J, Yuan S, Kok KH, To KK, Chan IH, Zhang AJ, Sit KY, Au WK, Yuen KY. Comparative replication and immune activation profiles of SARS-CoV-2 and SARS-CoV in human lungs: an ex vivo study with implications for the pathogenesis of COVID-19. Clin Infect Dis. 2020;71:1400–1409. doi: 10.1093/cid/ciaa410. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

