Clinical evaluation of rapid point-of-care antigen tests for diagnosis of SARS-CoV-2 infection
- PMID: 34021840
- PMCID: PMC8140309
- DOI: 10.1007/s10096-021-04274-7
Clinical evaluation of rapid point-of-care antigen tests for diagnosis of SARS-CoV-2 infection
Abstract
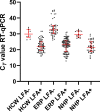
The RT-qPCR in respiratory specimens is the gold standard for diagnosing acute COVID-19 infections. However, this test takes considerable time before test results become available, thereby delaying patients from being diagnosed, treated, and isolated immediately. Rapid antigen tests could overcome this problem. In the first study, clinical performances of five rapid antigen tests were compared to RT-qPCR in upper respiratory specimens from 40 patients with positive and 40 with negative RTq-PCR results. In the second study, the rapid antigen test with one of the best test characteristics (Romed) was evaluated in a large prospective collection of upper respiratory specimens from 900 different COVID-19-suspected patients (300 emergency room patients, 300 nursing home patients, and 300 health care workers). Test specificities ranged from 87.5 to 100.0%, and test sensitivities from 55.0 to 80.0%. The clinical specificity of the Romed test was 99.8% (95% CI 98.9-100). Overall clinical sensitivity in the study population was 73.3% (95% CI 67.9-78.2), whereas sensitivity in the different patient groups varied from 65.3 to 86.7%. Sensitivity was 83.0 to 86.7% in patients with short duration of symptoms. In a population with a COVID-19 prevalence of 1%, the negative predictive value in all patients was 99.7%. There is a large variability in diagnostic performance between rapid antigen tests. The Romed rapid antigen test showed a good clinical performance in patients with high viral loads (RT-qPCR cycle threshold ≤30), which makes this antigen test suitable for rapid identification of COVID-19-infected health care workers and patients.
Keywords: COVID-19; Lateral flow immunoassay; POC test; Rapid antigen test; SARS-CoV-2.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Corman VM, Landt O, Kaiser M, Molenkamp R, Meijer A, Chu DKW, Bleicker T, Brünink S, Schneider J, Schmidt ML, Mulders DGJC, Haagmans BL, van der Veer B, van den Brink S, Wijsman L, Goderski G, Romette JL, Ellis J, Zambon M, Peiris M, Goossens H, Reusken C, Koopmans MPG, Drosten C. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Euro Surveill. 2020;2020:25(3). doi: 10.2807/1560-7917.ES.2020.25.3.2000045. - DOI - PMC - PubMed
-
- Albert E, Torres I, Bueno F, Huntley D, Molla E, Fernandez-Fuentes MA, Martínez M, Poujois S, Forque L, Valdivia A, de la Asuncion CS, Ferrer J, Colomina J, Navarro D. Field evaluation of a rapid antigen test (Panbio™ COVID-19 Ag Rapid Test Device) for COVID-19 diagnosis in primary healthcare centres. Clin Microbiol Infect. 2021;27:472.e7–472.e10. doi: 10.1016/j.cmi.2020.11.004. - DOI - PMC - PubMed
-
- Merino P, Guinea J, Munoz-Gallego I, Gonalez-Donapetry P, Galan JC, Antona N, Cilla G, Hernaez-Crespo S, Díaz-de Tuesta JL, Gual-de Torrella A, Gonzalez-Romo F, Escribano P, Sanchez-Castellano MA, Sota-Busselo M, Delgado-Iribarren A, García J, Canton R, Munoz P, Dolores Folgueira M, Cuenca-Estrella M, Oteo-Iglesias J (2021) Multicenter evaluation of the Panbio™ COVID-19 rapid antigendetection test for the diagnosis of SARS-CoV-2 infection. Clin Microbiol Infect. 10.1016/j.cmi.2021.02.001
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

