Prenatal muscle forces are necessary for vertebral segmentation and disc structure, but not for notochord involution in mice
- PMID: 34021906
- PMCID: PMC8268087
- DOI: 10.22203/eCM.v041a36
Prenatal muscle forces are necessary for vertebral segmentation and disc structure, but not for notochord involution in mice
Abstract
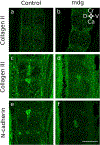
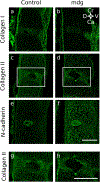
Embryonic muscle forces are necessary for normal vertebral development and spinal curvature, but their involvement in intervertebral disc (IVD) development remains unclear. The aim of the current study was to determine how muscle contractions affect (1) notochord involution and vertebral segmentation, and (2) IVD development including the mechanical properties and morphology, as well as collagen fibre alignment in the annulus fibrosus. Muscular dysgenesis (mdg) mice were harvested at three prenatal stages: at Theiler Stage (TS)22 when notochord involution starts, at TS24 when involution is complete, and at TS27 when the IVD is formed. Vertebral and IVD development were characterised using histology, immunofluorescence, and indentation testing. The results revealed that notochord involution and vertebral segmentation occurred independently of muscle contractions between TS22 and TS24. However, in the absence of muscle contractions, we found vertebral fusion in the cervical region at TS27, along with (i) a displacement of the nucleus pulposus towards the dorsal side, (ii) a disruption of the structural arrangement of collagen in the annulus fibrosus, and (iii) an increase in viscous behaviour of the annulus fibrosus. These findings emphasise the important role of mechanical forces during IVD development, and demonstrate a critical role of muscle loading during development to enable proper annulus fibrosus formation. They further suggest a need for mechanical loading in the creation of fibre-reinforced tissue engineering replacement IVDs as a therapy for IVD degeneration.
Figures






Similar articles
-
Suspension bioprinted whole intervertebral disc analogues enable regional stiffness- and hypoxia-regulated matrix secretion by primary human nucleus pulposus and annulus fibrosus cells.Acta Biomater. 2025 Jun 15;200:378-389. doi: 10.1016/j.actbio.2025.05.015. Epub 2025 May 7. Acta Biomater. 2025. PMID: 40339969
-
Individual Collagen Fibril Thickening and Stiffening of Annulus Fibrosus in Degenerative Intervertebral Disc.Spine (Phila Pa 1976). 2017 Oct 1;42(19):E1104-E1111. doi: 10.1097/BRS.0000000000002085. Spine (Phila Pa 1976). 2017. PMID: 28146016
-
Extracellular Matrix and Adhesion Molecule Gene Expression in the Normal and Injured Murine Intervertebral Disc.Am J Phys Med Rehabil. 2019 Jan;98(1):35-42. doi: 10.1097/PHM.0000000000001012. Am J Phys Med Rehabil. 2019. PMID: 30085932 Free PMC article.
-
Regeneration in Spinal Disease: Therapeutic Role of Hypoxia-Inducible Factor-1 Alpha in Regeneration of Degenerative Intervertebral Disc.Int J Mol Sci. 2021 May 17;22(10):5281. doi: 10.3390/ijms22105281. Int J Mol Sci. 2021. PMID: 34067899 Free PMC article. Review.
-
Mechanobiology of annulus fibrosus and nucleus pulposus cells in intervertebral discs.Cell Tissue Res. 2020 Mar;379(3):429-444. doi: 10.1007/s00441-019-03136-1. Epub 2019 Dec 17. Cell Tissue Res. 2020. PMID: 31844969 Review.
Cited by
-
Building a Co-ordinated Musculoskeletal System: The Plasticity of the Developing Skeleton in Response to Muscle Contractions.Adv Anat Embryol Cell Biol. 2023;236:81-110. doi: 10.1007/978-3-031-38215-4_4. Adv Anat Embryol Cell Biol. 2023. PMID: 37955772
-
Mechanical loading due to muscle movement regulates establishment of the collagen network in the developing murine skeleton.R Soc Open Sci. 2023 Oct 18;10(10):231023. doi: 10.1098/rsos.231023. eCollection 2023 Oct. R Soc Open Sci. 2023. PMID: 37859832 Free PMC article.
-
Lack of skeletal muscle contraction disrupts fibrous tissue morphogenesis in the developing murine knee.J Orthop Res. 2023 Oct;41(10):2305-2314. doi: 10.1002/jor.25659. Epub 2023 Jul 26. J Orthop Res. 2023. PMID: 37408453 Free PMC article.
References
-
- Ahmed S, Nowlan NC (2020) Initiation and emerging complexity of the collagen network during prenatal skeletal development. Eur Cell Mater 39: 136–155. - PubMed
-
- Antoniou J, Steffen T, Nelson F, Winterbottom N, Hollander AP, Poole RA, Aebi M, Alini M (1996) The human lumbar intervertebral disc: evidence for changes in the biosynthesis and denaturation of the extracellular matrix with growth, maturation, ageing, and degeneration. J Clin Invest 98: 996–1003. - PMC - PubMed
-
- Blecher R, Krief S, Galili T, Biton IE, Stern T, Assaraf E, Levanon D, Appel E, Anekstein Y, Agar G, Groner Y, Zelzer E (2017) The proprioceptive system masterminds spinal alignment: insight into the mechanism of scoliosis. Dev Cell 42: 388–399. - PubMed
Web reference
Additional References
-
- Flück M, Giraud M-N, Tunç V, Chiquet M (2003) Tensile stress-dependent collagen XII and fibronectin production by fibroblasts requires separate pathways, Biochim Biophys Acta 1593: 239–248. - PubMed
-
- Lindholm ME, Rundqvist H (2016) Skeletal muscle hypoxia-inducible factor-1 and exercise. Exp Physiol 101: 28–32. - PubMed
-
- Sato S, Kimura A, Ozdemir J, Asou Y, Miyazaki M, Jinno T, Ae K, Liu X, Osaki M, Takeuchi Y, Fukumoto S, Kawaguchi H, Haro H, Shinomiya K, Karsenty G, Takeda S (2008) The distinct role of the Runx proteins in chondrocyte differentiation and intervertebral disc degeneration: findings in murine models and in human disease. Arthritis Rheum 58: 2764–2775. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources