Post-transplant cyclophosphamide limits reactive donor T cells and delays the development of graft-versus-host disease in a humanized mouse model
- PMID: 34021907
- PMCID: PMC8442241
- DOI: 10.1111/imm.13374
Post-transplant cyclophosphamide limits reactive donor T cells and delays the development of graft-versus-host disease in a humanized mouse model
Abstract
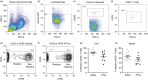
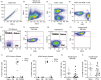
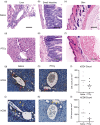
Graft-versus-host disease (GVHD) is a major complication of allogeneic haematopoietic stem cell transplantation (allo-HSCT) that develops when donor T cells in the graft become reactive against the host. Post-transplant cyclophosphamide (PTCy) is increasingly used in mismatched allo-HSCT, but how PTCy impacts donor T cells and reduces GVHD is unclear. This study aimed to determine the effect of PTCy on reactive human donor T cells and GVHD development in a preclinical humanized mouse model. Immunodeficient NOD-scid-IL2Rγnull mice were injected intraperitoneally (i.p.) with 20 × 106 human peripheral blood mononuclear cells stained with carboxyfluorescein succinimidyl ester (CFSE) (day 0). Mice were subsequently injected (i.p.) with PTCy (33 mg kg-1 ) (PTCy-mice) or saline (saline-mice) (days 3 and 4). Mice were assessed for T-cell depletion on day 6 and monitored for GVHD for up to 10 weeks. Flow cytometric analysis of livers at day 6 revealed lower proportions of reactive (CFSElow ) human (h) CD3+ T cells in PTCy-mice compared with saline-mice. Over 10 weeks, PTCy-mice showed reduced weight loss and clinical GVHD, with prolonged survival and reduced histological liver GVHD compared with saline-mice. PTCy-mice also demonstrated increased splenic hCD4+ :hCD8+ T-cell ratios and reduced splenic Tregs (hCD4+ hCD25+ hCD127lo ) compared with saline-mice. This study demonstrates that PTCy reduces GVHD in a preclinical humanized mouse model. This corresponded to depletion of reactive human donor T cells, but fewer human Tregs.
Keywords: graft-versus-host disease; humanized mice; post-transplant cyclophosphamide; reactive T cells; regulatory T cells; xenogeneic.
© 2021 John Wiley & Sons Ltd.
Conflict of interest statement
Sam R. Adhikary, Peter Cuthbertson, Leigh Nicholson, Katrina M. Bird, Chloe Sligar, Min Hu, Philip J O'Connell, Ronald Sluyter, Stephen I. Alexander and Debbie Watson declare that they have no conflict of interest.
Figures

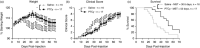



Similar articles
-
Tocilizumab increases regulatory T cells, reduces natural killer cells and delays graft-versus-host disease development in humanized mice treated with post-transplant cyclophosphamide.Immunol Cell Biol. 2023 Aug;101(7):639-656. doi: 10.1111/imcb.12652. Epub 2023 May 16. Immunol Cell Biol. 2023. PMID: 37191045
-
Post-Transplant Cyclophosphamide Combined with Brilliant Blue G Reduces Graft-versus-Host Disease without Compromising Graft-versus-Leukaemia Immunity in Humanised Mice.Int J Mol Sci. 2024 Feb 1;25(3):1775. doi: 10.3390/ijms25031775. Int J Mol Sci. 2024. PMID: 38339054 Free PMC article.
-
A single-nucleotide polymorphism in the human ENTPD1 gene encoding CD39 is associated with worsened graft-versus-host disease in a humanized mouse model.Immunol Cell Biol. 2020 May;98(5):397-410. doi: 10.1111/imcb.12328. Epub 2020 Apr 16. Immunol Cell Biol. 2020. PMID: 32181525
-
[Current state of graft-versus-host disease prophylaxis with PTCy for allogeneic hematopoietic stem cell transplantation].Rinsho Ketsueki. 2024;65(5):391-400. doi: 10.11406/rinketsu.65.391. Rinsho Ketsueki. 2024. PMID: 38825519 Review. Japanese.
-
Systematic overview of HLA-matched allogeneic hematopoietic cell transplantation with post-transplantation cyclophosphamide.Int J Hematol. 2022 Oct;116(4):465-481. doi: 10.1007/s12185-022-03428-3. Epub 2022 Aug 5. Int J Hematol. 2022. PMID: 35930118
Cited by
-
Purinergic Signalling in Allogeneic Haematopoietic Stem Cell Transplantation and Graft-versus-Host Disease.Int J Mol Sci. 2021 Aug 3;22(15):8343. doi: 10.3390/ijms22158343. Int J Mol Sci. 2021. PMID: 34361109 Free PMC article. Review.
-
Graft-Versus-Host Disease Mouse Models: A Clinical-Translational Perspective.Methods Mol Biol. 2025;2907:1-56. doi: 10.1007/978-1-0716-4430-0_1. Methods Mol Biol. 2025. PMID: 40100591 Review.
-
Post-transplant cyclophosphamide prevents xenogeneic graft-versus-host disease while depleting proliferating regulatory T cells.iScience. 2023 Jan 31;26(3):106085. doi: 10.1016/j.isci.2023.106085. eCollection 2023 Mar 17. iScience. 2023. PMID: 36843851 Free PMC article.
-
Insights into mechanisms of graft-versus-host disease through humanised mouse models.Biosci Rep. 2022 Sep 30;42(9):BSR20211986. doi: 10.1042/BSR20211986. Biosci Rep. 2022. PMID: 35993192 Free PMC article. Review.
-
Comment on "MSCohi-O lenses for long-term retention of mesenchymal stem cells on ocular surface as a therapeutic approach for chronic ocular graft-versus-host disease".Stem Cell Reports. 2025 Mar 11;20(3):102401. doi: 10.1016/j.stemcr.2025.102401. Epub 2025 Feb 6. Stem Cell Reports. 2025. PMID: 39919753 Free PMC article. No abstract available.
References
-
- Zhang Y, Joe G, Hexner E, Zhu J, Emerson SG. Alloreactive memory T cells are responsible for the persistence of graft‐versus‐host disease. J Immunol. 2005;174:3051–8. - PubMed
-
- Gendelman M, Yassai M, Tivol E, Krueger A, Gorski J, Drobyski WR. Selective elimination of alloreactive donor T cells attenuates graft‐versus‐host disease and enhances T‐cell reconstitution. Biol Blood Marrow Transplant. 2003;9:742–52. - PubMed
-
- Fowler DH. Shared biology of GVHD and GVT effects: potential methods of separation. Crit Rev Oncol Hematol. 2006;57:225–44. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

