Therapeutic RNA interference: A novel approach to the treatment of primary hyperoxaluria
- PMID: 34022071
- PMCID: PMC9291495
- DOI: 10.1111/bcp.14925
Therapeutic RNA interference: A novel approach to the treatment of primary hyperoxaluria
Abstract
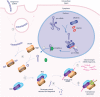
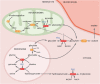
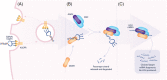
RNA interference (RNAi) is a natural biological pathway that inhibits gene expression by targeted degradation or translational inhibition of cytoplasmic mRNA by the RNA induced silencing complex. RNAi has long been exploited in laboratory research to study the biological consequences of the reduced expression of a gene of interest. More recently RNAi has been demonstrated as a therapeutic avenue for rare metabolic diseases. This review presents an overview of the cellular RNAi machinery as well as therapeutic RNAi design and delivery. As a clinical example we present primary hyperoxaluria, an ultrarare inherited disease of increased hepatic oxalate production which leads to recurrent calcium oxalate kidney stones. In the most common form of the disease (Type 1), end-stage kidney disease occurs in childhood or young adulthood, often necessitating combined kidney and liver transplantation. In this context we discuss nedosiran (Dicerna Pharmaceuticals, Inc.) and lumasiran (Alnylam Pharmaceuticals), which are both novel RNAi therapies for primary hyperoxaluria that selectively reduce hepatic expression of lactate dehydrogenase and glycolate oxidase respectively, reducing hepatic oxalate production and urinary oxalate levels. Finally, we consider future optimizations advances in RNAi therapies.
Keywords: RNA interference; calcium oxalate; end-stage renal disease; glycolate oxidase; hyperoxaluria; kidney stones; lactate dehydrogenase; micro-RNA; primary hyperoxaluria; small interfering RNAs.
© 2021 The Authors. British Journal of Clinical Pharmacology published by John Wiley & Sons Ltd on behalf of British Pharmacological Society.
Conflict of interest statement
C.L. and B.D.B. are employees of Dicerna Pharmaceuticals, which is developing siRNAs as therapeutics, including nedosiran. T.A.F. is the Site Principle Investigator at the Royal Children's Hospital in Melbourne, Australia supervising Dicerna (nedosiran) and Alnylam (lumasiran) clinical trials.
Figures
Similar articles
-
Specific Inhibition of Hepatic Lactate Dehydrogenase Reduces Oxalate Production in Mouse Models of Primary Hyperoxaluria.Mol Ther. 2018 Aug 1;26(8):1983-1995. doi: 10.1016/j.ymthe.2018.05.016. Epub 2018 Jun 15. Mol Ther. 2018. PMID: 29914758 Free PMC article.
-
Phase 1/2 Study of Lumasiran for Treatment of Primary Hyperoxaluria Type 1: A Placebo-Controlled Randomized Clinical Trial.Clin J Am Soc Nephrol. 2021 Jul;16(7):1025-1036. doi: 10.2215/CJN.14730920. Epub 2021 May 13. Clin J Am Soc Nephrol. 2021. PMID: 33985991 Free PMC article. Clinical Trial.
-
Lumasiran, an RNAi Therapeutic for Primary Hyperoxaluria Type 1.N Engl J Med. 2021 Apr 1;384(13):1216-1226. doi: 10.1056/NEJMoa2021712. N Engl J Med. 2021. PMID: 33789010 Clinical Trial.
-
New therapeutics for primary hyperoxaluria type 1.Curr Opin Nephrol Hypertens. 2022 Jul 1;31(4):344-350. doi: 10.1097/MNH.0000000000000790. Epub 2022 Mar 9. Curr Opin Nephrol Hypertens. 2022. PMID: 35266883 Free PMC article. Review.
-
Human glyoxylate metabolism revisited: New insights pointing to multi-organ involvement with implications for siRNA-based therapies in primary hyperoxaluria.J Inherit Metab Dis. 2025 Jan;48(1):e12817. doi: 10.1002/jimd.12817. Epub 2024 Nov 24. J Inherit Metab Dis. 2025. PMID: 39582099 Free PMC article. Review.
Cited by
-
A molecular journey on the pathogenesis of primary hyperoxaluria.Curr Opin Nephrol Hypertens. 2024 Jul 1;33(4):398-404. doi: 10.1097/MNH.0000000000000987. Epub 2024 Apr 11. Curr Opin Nephrol Hypertens. 2024. PMID: 38602143 Free PMC article. Review.
-
Hydration and Nephrolithiasis in Pediatric Populations: Specificities and Current Recommendations.Nutrients. 2023 Feb 1;15(3):728. doi: 10.3390/nu15030728. Nutrients. 2023. PMID: 36771434 Free PMC article. Review.
-
Oxalate (dys)Metabolism: Person-to-Person Variability, Kidney and Cardiometabolic Toxicity.Genes (Basel). 2023 Aug 29;14(9):1719. doi: 10.3390/genes14091719. Genes (Basel). 2023. PMID: 37761859 Free PMC article. Review.
-
Primary hyperoxaluria type 1 diagnosis in adult dialysis patients: prediction model assessment in a group of Italian patients.J Nephrol. 2025 Jun 4. doi: 10.1007/s40620-025-02243-3. Online ahead of print. J Nephrol. 2025. PMID: 40464871
-
The real world experience of pediatric primary hyperoxaluria patients in the PEDSnet clinical research network.Eur J Pediatr. 2023 Sep;182(9):4027-4036. doi: 10.1007/s00431-023-05077-y. Epub 2023 Jul 1. Eur J Pediatr. 2023. PMID: 37392234
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

