Seroprevalence of anti-SARS-CoV-2 antibodies in Iquitos, Peru in July and August, 2020: a population-based study
- PMID: 34022148
- PMCID: PMC8133769
- DOI: 10.1016/S2214-109X(21)00173-X
Seroprevalence of anti-SARS-CoV-2 antibodies in Iquitos, Peru in July and August, 2020: a population-based study
Abstract
Background: Detection of anti-SARS-CoV-2 antibodies among people at risk of infection is crucial for understanding both the past transmission of COVID-19 and vulnerability of the population to continuing transmission and, when done serially, the intensity of ongoing transmission over an interval in a community. We aimed to estimate the seroprevalence of COVID-19 in a representative population-based cohort in Iquitos, one of the regions with the highest mortality rates from COVID-19 in Peru, where a devastating number of cases occurred in March, 2020.
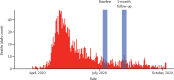
Methods: We did a population-based study of SARS-CoV-2 transmission in Iquitos at two timepoints: July 13-18, 2020 (baseline), and Aug 13-18, 2020 (1-month follow-up). We obtained a geographically stratified representative sample of the city population using the 2017 census data, which was updated on Jan 20, 2020. We included people who were inhabitants of Iquitos since COVID-19 was identified in Peru (March 6, 2020) or earlier. We excluded people living in institutions, people receiving any pharmacological treatment for COVID-19, people with any contraindication for phlebotomy, and health workers or individuals living with an active health worker. We tested each participant for IgG and IgM anti-SARS-CoV-2 antibodies using the COVID-19 IgG/IgM Rapid Test (Zhejiang Orient Gene Biotech, China). We used survey analysis methods to estimate seroprevalence accounting for the sampling design effect and test performance characteristics.
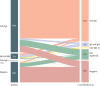
Findings: We identified 726 eligible individuals and enrolled a total of 716 participants (99%), distributed across 40 strata (four districts, two sexes, and five age groups). We excluded ten individuals who: did not have consent from a parent or legal representative (n=3), had moved to Iquitos after March 6, 2020 (n=3), were in transit (n=2), or had respiratory symptoms (n=1). After adjusting for the study sampling effects and sensitivity and specificity of the test, we estimated a seroprevalence of 70% (95% CI 67-73) at baseline and 66% (95% CI 62-70) at 1 month of follow-up, with a test-retest positivity of 65% (95% CI 61-68), and an incidence of new exposures of 2% (95% CI 1-3). We observed significant differences in the seroprevalence between age groups, with participants aged 18-29 years having lower seroprevalence than those aged younger than 12 years (prevalence ratio 0·85 [95% CI 0·73-0·98]; p=0·029).
Interpretation: After the first epidemic peak, Iquitos had one of the highest rates of seroprevalence of anti-SARS-CoV-2 antibodies worldwide. Nevertheless, the city experienced a second wave starting in January, 2021, probably due to the emergence of the SARS-CoV-2 P1 variant, which has shown higher transmissibility and reinfection rates.
Funding: Dirección Regional de Salud de Loreto (DIRESA), Loreto, Peru.
Translation: For the Spanish translation of the abstract see Supplementary Materials section.
Copyright © 2021 The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY 4.0 license. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of interests We declare no competing interests.
Figures
Comment in
-
Reaching the theoretical herd immunity threshold in Iquitos, Peru: are seroprevalence data enough?Lancet Glob Health. 2021 Jul;9(7):e881-e882. doi: 10.1016/S2214-109X(21)00203-5. Epub 2021 May 19. Lancet Glob Health. 2021. PMID: 34022149 Free PMC article. No abstract available.
References
-
- Aquino M, Garrison C. Peru records first confirmed case of coronavirus, President Vizcarra says. March 6, 2020. https://www.reuters.com/article/us-health-coronavirus-peru-idUSKBN20T1S9
-
- Ministry of Health of Peru Sala situacional COVID-19 Peru. 2020. https://covid19.minsa.gob.pe/sala_situacional.asp
-
- WHO . World Health Organization; Geneva: 2020. Population-based age-stratified seroepidemiological investigation protocol for COVID-19 virus infection.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

