Fine Sampling of Sequence Space for Membrane Protein Structural Biology
- PMID: 34022208
- PMCID: PMC8286341
- DOI: 10.1016/j.jmb.2021.167055
Fine Sampling of Sequence Space for Membrane Protein Structural Biology
Abstract
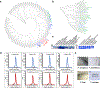
We describe an enhancement of traditional genomics-based approaches to improve the success of structure determination of membrane proteins. Following a broad screen of sequence space to identify initial expression-positive targets, we employ a second step to select orthologs with closely related sequences to these hits. We demonstrate that a greater percentage of these latter targets express well and are stable in detergent, increasing the likelihood of identifying candidates that will ultimately yield structural information.
Keywords: high throughput biology; integral membrane proteins; protein expression; protein purification; structural biology; structural genomics.
Copyright © 2021 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Competing Interests The authors declare no competing interests.
Figures


Similar articles
-
Structural genomics plucks high-hanging membrane proteins.Curr Opin Struct Biol. 2012 Jun;22(3):326-32. doi: 10.1016/j.sbi.2012.05.002. Epub 2012 May 21. Curr Opin Struct Biol. 2012. PMID: 22622032 Free PMC article. Review.
-
Expression of Prokaryotic Integral Membrane Proteins in E. coli.Methods Mol Biol. 2017;1586:265-278. doi: 10.1007/978-1-4939-6887-9_17. Methods Mol Biol. 2017. PMID: 28470611
-
Backbone structure of a small helical integral membrane protein: A unique structural characterization.Protein Sci. 2009 Jan;18(1):134-46. doi: 10.1002/pro.24. Protein Sci. 2009. PMID: 19177358 Free PMC article.
-
Target selection and deselection at the Berkeley Structural Genomics Center.Proteins. 2006 Feb 1;62(2):356-70. doi: 10.1002/prot.20674. Proteins. 2006. PMID: 16276528
-
Structural genomics for membrane proteins.Cell Mol Life Sci. 2006 Nov;63(22):2597-607. doi: 10.1007/s00018-006-6252-y. Cell Mol Life Sci. 2006. PMID: 17013556 Free PMC article. Review.
References
-
- Tate CG. A crystal clear solution for determining G-protein-coupled receptor structures. Trends Biochem Sci. 2012;37:343–52. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

