A simplified alternative diagnostic algorithm for SARS-CoV-2 suspected symptomatic patients and confirmed close contacts (asymptomatic): A consensus of Latin American experts
- PMID: 34022333
- PMCID: PMC8133827
- DOI: 10.1016/j.ijid.2021.05.011
A simplified alternative diagnostic algorithm for SARS-CoV-2 suspected symptomatic patients and confirmed close contacts (asymptomatic): A consensus of Latin American experts
Abstract
Introduction: Latin America accounts for one-quarter of global COVID-19 cases and one-third of deaths. Inequalities in the region lead to barriers to the best use of diagnostic tests during the pandemic. There is a need for simplified guidelines that consider the region's limited health resources, international guidelines, medical literature, and local expertise.
Methods: Using a modified Delphi method, 9 experts from Latin American countries developed a simplified algorithm for COVID-19 diagnosis on the basis of their answers to 24 questions related to diagnostic settings, and discussion of the literature and their experiences.
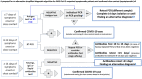
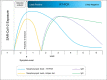
Results: The algorithm considers 3 timeframes (≤7 days, 8-13 days, and ≥14 days) and presents diagnostic options for each. SARS-CoV-2 real- time reverse transcription-polymerase chain reaction is the test of choice from day 1 to 14 after symptom onset or close contact, although antigen testing may be used in specific circumstances, from day 5 to 7. Antibody assays may be used for confirmation, usually after day 14; however, if clinical suspicion is very high, but other tests are negative, these assays may be used as an adjunct to decision-making from day 8 to 13.
Conclusion: The proposed algorithm aims to support COVID-19 diagnosis decision-making in Latin America.
Keywords: Algorithm; COVID-19; Diagnosis; Latin America; SARS-CoV- 2.
Copyright © 2021. Published by Elsevier Ltd.
Figures
References
-
- 2019-nCoV Working Group. Communicable Diseases Network Australia . 2020. Coronavirus disease 2019 (COVID-19) CDNA national guidelines for public health units.https://www1.health.gov.au/internet/main/publishing.nsf/Content/7A8654A8... [Accessed 8 November 2020]
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

