Classifying information-sharing methods
- PMID: 34022810
- PMCID: PMC8140466
- DOI: 10.1186/s12874-021-01292-z
Classifying information-sharing methods
Abstract
Background: Sparse relative effectiveness evidence is a frequent problem in Health Technology Assessment (HTA). Where evidence directly pertaining to the decision problem is sparse, it may be feasible to expand the evidence-base to include studies that relate to the decision problem only indirectly: for instance, when there is no evidence on a comparator, evidence on other treatments of the same molecular class could be used; similarly, a decision on children may borrow-strength from evidence on adults. Usually, in HTA, such indirect evidence is either included by ignoring any differences ('lumping') or not included at all ('splitting'). However, a range of more sophisticated methods exists, primarily in the biostatistics literature. The objective of this study is to identify and classify the breadth of the available information-sharing methods.
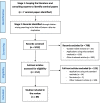
Methods: Forwards and backwards citation-mining techniques were used on a set of seminal papers on the topic of information-sharing. Papers were included if they specified (network) meta-analytic methods for combining information from distinct populations, interventions, outcomes or study-designs.
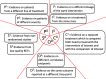
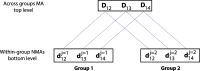
Results: Overall, 89 papers were included. A plethora of evidence synthesis methods have been used for information-sharing. Most papers (n=79) described methods that shared information on relative treatment effects. Amongst these, there was a strong emphasis on methods for information-sharing across multiple outcomes (n=42) and treatments (n=25), with fewer papers focusing on study-designs (n=23) or populations (n=8). We categorise and discuss the methods under four 'core' relationships of information-sharing: functional, exchangeability-based, prior-based and multivariate relationships, and explain the assumptions made within each of these core approaches.
Conclusions: This study highlights the range of information-sharing methods available. These methods often impose more moderate assumptions than lumping or splitting. Hence, the degree of information-sharing that they impose could potentially be considered more appropriate. Our identification of four 'core' methods of information-sharing allows for an improved understanding of the assumptions underpinning the different methods. Further research is required to understand how the methods differ in terms of the strength of sharing they impose and the implications of this for health care decisions.
Keywords: Borrowing-strength; Indirect evidence; Information-sharing; Meta-analysis; Network meta-analysis.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

Similar articles
-
The future of Cochrane Neonatal.Early Hum Dev. 2020 Nov;150:105191. doi: 10.1016/j.earlhumdev.2020.105191. Epub 2020 Sep 12. Early Hum Dev. 2020. PMID: 33036834
-
The Effectiveness of Integrated Care Pathways for Adults and Children in Health Care Settings: A Systematic Review.JBI Libr Syst Rev. 2009;7(3):80-129. doi: 10.11124/01938924-200907030-00001. JBI Libr Syst Rev. 2009. PMID: 27820426
-
Promoting and supporting self-management for adults living in the community with physical chronic illness: A systematic review of the effectiveness and meaningfulness of the patient-practitioner encounter.JBI Libr Syst Rev. 2009;7(13):492-582. doi: 10.11124/01938924-200907130-00001. JBI Libr Syst Rev. 2009. PMID: 27819974
-
Modelling approaches for histology-independent cancer drugs to inform NICE appraisals: a systematic review and decision-framework.Health Technol Assess. 2021 Dec;25(76):1-228. doi: 10.3310/hta25760. Health Technol Assess. 2021. PMID: 34990339
-
Comparison of conference abstracts and presentations with full-text articles in the health technology assessments of rapidly evolving technologies.Health Technol Assess. 2006 Feb;10(5):iii-iv, ix-145. doi: 10.3310/hta10050. Health Technol Assess. 2006. PMID: 16487455 Review.
Cited by
-
Multi-indication Evidence Synthesis in Oncology Health Technology Assessment: Meta-analysis Methods and Their Application to a Case Study of Bevacizumab.Med Decis Making. 2025 Jan;45(1):17-33. doi: 10.1177/0272989X241295665. Epub 2024 Nov 18. Med Decis Making. 2025. PMID: 39555661 Free PMC article.
-
Challenges around quantifying uncertainty in a holistic approach to hard-to-heal wound management: Health economic perspective.Int Wound J. 2023 Mar;20(3):792-798. doi: 10.1111/iwj.13924. Epub 2022 Sep 8. Int Wound J. 2023. PMID: 36073595 Free PMC article.
-
Comparison of Bayesian methods for incorporating adult clinical trial data to improve certainty of treatment effect estimates in children.PLoS One. 2023 Jun 15;18(6):e0281791. doi: 10.1371/journal.pone.0281791. eCollection 2023. PLoS One. 2023. PMID: 37319173 Free PMC article.
-
Problematic meta-analyses: Bayesian and frequentist perspectives on combining randomized controlled trials and non-randomized studies.BMC Med Res Methodol. 2024 Apr 27;24(1):99. doi: 10.1186/s12874-024-02215-4. BMC Med Res Methodol. 2024. PMID: 38678213 Free PMC article.
-
Structured expert elicitation to inform long-term survival extrapolations using alternative parametric distributions: a case study of CAR T therapy for relapsed/ refractory multiple myeloma.BMC Med Res Methodol. 2022 Oct 15;22(1):272. doi: 10.1186/s12874-022-01745-z. BMC Med Res Methodol. 2022. PMID: 36243687 Free PMC article.
References
-
- World Health Organization. WHO HTA Definition (EB 134/30). 2018. http://www.who.int/health-technology-assessment/about/Defining/en/. Accessed 1 Apr 2021.
-
- Claxton K, Martin S, Soares M, Rice N, Spackman E, Hinde S, Devlin N, Smith PC, Sculpher M. Methods for the estimation of the National Institute for Health and Care Excellence cost-effectiveness threshold. Health Technol Assess (Winchester, England) 2015;19(14):1–503. doi: 10.3310/hta19140. - DOI - PMC - PubMed
-
- Drummond MF, Sculpher MJ, Claxton K, Stoddart GL, Torrance GW. Methods for the Economic Evaluation of Health Care Programmes. Oxford: Oxford University Press; 2015.
-
- Briggs A, Claxton K, Sculpher MJ. Decision Modelling for Health Economic Evaluation. Oxford: Oxford University Press; 2006.
-
- Centre for Reviews and Dissemination. Systematic reviews. CRD‘s guidance for undertaking reviews in health care. Centre for Reviews and Dissemination. 2009. https://www.york.ac.uk/media/crd/Systematic_Reviews.pdf.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

