Annexin A1 protects against cerebral ischemia-reperfusion injury by modulating microglia/macrophage polarization via FPR2/ALX-dependent AMPK-mTOR pathway
- PMID: 34022892
- PMCID: PMC8140477
- DOI: 10.1186/s12974-021-02174-3
Annexin A1 protects against cerebral ischemia-reperfusion injury by modulating microglia/macrophage polarization via FPR2/ALX-dependent AMPK-mTOR pathway
Abstract
Background: Cerebral ischemia-reperfusion (I/R) injury is a major cause of early complications and unfavorable outcomes after endovascular thrombectomy (EVT) therapy in patients with acute ischemic stroke (AIS). Recent studies indicate that modulating microglia/macrophage polarization and subsequent inflammatory response may be a potential adjunct therapy to recanalization. Annexin A1 (ANXA1) exerts potent anti-inflammatory and pro-resolving properties in models of cerebral I/R injury. However, whether ANXA1 modulates post-I/R-induced microglia/macrophage polarization has not yet been fully elucidated.
Methods: We retrospectively collected blood samples from AIS patients who underwent successful recanalization by EVT and analyzed ANXA1 levels longitudinally before and after EVT and correlation between ANXA1 levels and 3-month clinical outcomes. We also established a C57BL/6J mouse model of transient middle cerebral artery occlusion/reperfusion (tMCAO/R) and an in vitro model of oxygen-glucose deprivation and reoxygenation (OGD/R) in BV2 microglia and HT22 neurons to explore the role of Ac2-26, a pharmacophore N-terminal peptide of ANXA1, in regulating the I/R-induced microglia/macrophage activation and polarization.
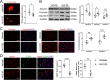
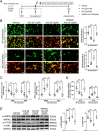
Results: The baseline levels of ANXA1 pre-EVT were significantly lower in 23 AIS patients, as compared with those of healthy controls. They were significantly increased to the levels found in controls 2-3 days post-EVT. The increased post-EVT levels of ANXA1 were positively correlated with 3-month clinical outcomes. In the mouse model, we then found that Ac2-26 administered at the start of reperfusion shifted microglia/macrophage polarization toward anti-inflammatory M2-phenotype in ischemic penumbra, thus alleviating blood-brain barrier leakage and neuronal apoptosis and improving outcomes at 3 days post-tMCAO/R. The protection was abrogated when mice received Ac2-26 together with WRW4, which is a specific antagonist of formyl peptide receptor type 2/lipoxin A4 receptor (FPR2/ALX). Furthermore, the interaction between Ac2-26 and FPR2/ALX receptor activated the 5' adenosine monophosphate-activated protein kinase (AMPK) and inhibited the downstream mammalian target of rapamycin (mTOR). These in vivo findings were validated through in vitro experiments.
Conclusions: Ac2-26 modulates microglial/macrophage polarization and alleviates subsequent cerebral inflammation by regulating the FPR2/ALX-dependent AMPK-mTOR pathway. It may be investigated as an adjunct strategy for clinical prevention and treatment of cerebral I/R injury after recanalization. Plasma ANXA1 may be a potential biomarker for outcomes of AIS patients receiving EVT.
Keywords: Annexin A1; Cerebral ischemia-reperfusion injury; Endovascular thrombectomy; Formyl peptide receptor 2; Microglial/macrophage polarization; Neuroinflammation.
Conflict of interest statement
The authors declare no competing interests.
Figures





References
-
- Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, Biller J, Brown M, Demaerschalk BM, Hoh B, Jauch EC, Kidwell CS, Leslie-Mazwi TM, Ovbiagele B, Scott PA, Sheth KN, Southerland AM, Summers DV, Tirschwell DL, on behalf of the American Heart Association Stroke Council Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2019;50(12):e344–e418. doi: 10.1161/STR.0000000000000211. - DOI - PubMed
-
- Xu H, Jia B, Huo X, Mo D, Ma N, Gao F, Yang M, Miao Z. Predictors of futile recanalization after endovascular treatment in patients with acute ischemic stroke in a multicenter registry study. J Stroke Cerebrovasc Dis. 2020;29(10):105067. doi: 10.1016/j.jstrokecerebrovasdis.2020.105067. - DOI - PubMed
MeSH terms
Substances
Grants and funding
- 82001317/National Natural Science Foundation of China
- 81801231/National Natural Science Foundation of China
- 81671380/National Natural Science Foundation of China
- 2016YFC1301703/National Key Scientific Instrument and Equipment Development Projects of China (CN)
- D161100003816002/Beijing Science and Technology Planning Project
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

