Extracellular vesicles-based pre-targeting strategy enables multi-modal imaging of orthotopic colon cancer and image-guided surgery
- PMID: 34022897
- PMCID: PMC8141172
- DOI: 10.1186/s12951-021-00888-3
Extracellular vesicles-based pre-targeting strategy enables multi-modal imaging of orthotopic colon cancer and image-guided surgery
Abstract
Backgroud: Colon cancer contributes to high mortality rates as the result of incomplete resection in tumor surgery. Multimodal imaging can provide preoperative evaluation and intraoperative image-guiding. As biocompatible nanocarriers, extracellular vesicles hold great promise for multimodal imaging. In this study, we aim to synthesized an extracellular vesicles-based nanoprobe to visualize colon cancer with positron-emission tomography/computed tomography (PET/CT) and near-infrared fluorescence (NIRF) imaging, and investigated its utility in image-guided surgery of colon cancer in animal models.
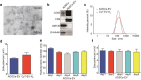
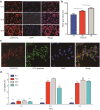
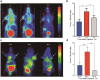
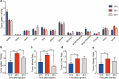
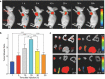
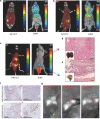
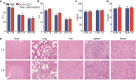
Results: Extracellular vesicles were successfully isolated from adipose-derived stem cells (ADSCs), and their membrane vesicles were observed under TEM. DLS detected that the hydrodynamic diameters of the extracellular vesicles were approximately 140 nm and the zeta potential was - 7.93 ± 0.24 mV. Confocal microscopy showed that extracellular vesicles had a strong binding ability to tumor cells. A click chemistry-based pre-targeting strategy was used to achieve PET imaging in vivo. PET images and the biodistribution results showed that the best pre-targeting time was 20 h, and the best imaging time was 2 h after the injection of 68 Ga-L-NETA-DBCO. The NIRF images showed that the tumor had clear images at all time points after administration of nanoparticles and the Tumor/Muscle ratio peaked at 20 h after injection. Our data also showed that both PET/CT and NIRF imaging clearly visualized the orthotopic colon cancer models, providing preoperative evaluation. Under real-time NIRF imaging, the tumor location and tumor boundary could be clearly observed.
Conclusions: In brief, this novel nanoprobe may be useful for multi-modal imaging of colon cancer and NIRF image-guided surgery. More importantly, this study provides a new possibility for clinical application of extracellular vesicles as nanocarriers.
Keywords: Extracellular vesicles; Image-guided surgery; Multimodal imaging; NIRF; PET/CT.
Conflict of interest statement
The authors declare that there are no conflicts of interest regarding the publication of this paper.
Figures

References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

