Fasciola gigantica tegumental calcium-binding EF-hand protein 4 exerts immunomodulatory effects on goat monocytes
- PMID: 34022913
- PMCID: PMC8141160
- DOI: 10.1186/s13071-021-04784-5
Fasciola gigantica tegumental calcium-binding EF-hand protein 4 exerts immunomodulatory effects on goat monocytes
Abstract
Background: The liver fluke Fasciola gigantica secretes excretory-secretory proteins during infection to mediate its interaction with the host. In this study, we investigated the immunomodulatory effects of a recombinant tegumental calcium-binding EF-hand protein 4 of F. gigantica (rFg-CaBP4) on goat monocytes.
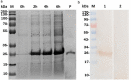
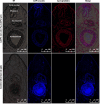
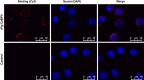
Methods: The rFg-CaBP4 protein was induced and purified by affinity chromatography. The immunogenic reaction of rFg-CaBP4 against specific antibodies was detected through western blot analysis. The binding of rFg-CaBP4 on surface of goat monocytes was visualized by immunofluorescence assay. The localization of CaBP4 within adult fluke structure was detected by immunohistochemical analysis. The cytokine transcription levels in response to rFg-CaBP4 were examined using ABI 7500 real-time PCR system. The expression of the major histocompatibility complex (MHC) class-II molecule (MHC-II) in response to rFg-CaBP4 protein was analyzed using Flow cytometry.
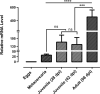
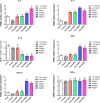
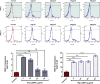
Results: The isopropyl-ß-D-thiogalactopyranoside-induced rFg-CaBP4 protein reacted with rat sera containing anti-rFg-CaBP4 polyclonal antibodies in a western blot analysis. The adhesion of rFg-CaBP4 to monocytes was visualized by immunofluorescence and laser scanning confocal microscopy. Immunohistochemical analysis localized native CaBP4 to the oral sucker, pharynx, genital pore, acetabulum and tegument of adult F. gigantica. Co-incubation of rFg-CaBP4 with concanavalin A-stimulated monocytes increased the transcription levels of interleukin (IL)-2, IL-4, interferon gamma and transforming growth factor-β. However, a reduction in the expression of IL-10 and no change in the expression of tumor necrosis factor-α were detected. Additionally, rFg-CaBP4-treated monocytes exhibited a marked increase in the expression of the major histocompatibility complex (MHC) class-II molecule (MHC-II) and a decrease in MHC-I expression, in a dose-dependent manner.
Conclusions: These findings provide additional evidence that calcium-binding EF-hand proteins play roles in host-parasite interaction. Further characterization of the immunomodulatory role of rFg-CaBP4 should expand our understanding of the strategies used by F. gigantica to evade the host immune responses.
Keywords: Fasciola gigantica; Host-parasite interactions; Immune responses; Monocytes; Tegumental calcium-binding EF-hand protein 4.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

Similar articles
-
Immune modulation of goat monocytes by Fasciola gigantica Legumain-1 protein (Fg-LGMN-1).Exp Parasitol. 2024 Jan;256:108671. doi: 10.1016/j.exppara.2023.108671. Epub 2023 Dec 9. Exp Parasitol. 2024. PMID: 38081528
-
The pervasive effects of recombinant Fasciola gigantica Ras-related protein Rab10 on the functions of goat peripheral blood mononuclear cells.Parasit Vectors. 2018 Nov 6;11(1):579. doi: 10.1186/s13071-018-3148-2. Parasit Vectors. 2018. PMID: 30400957 Free PMC article.
-
Analysis of a calcium-binding EF-hand protein family in Fasciola gigantica.Exp Parasitol. 2012 Apr;130(4):364-73. doi: 10.1016/j.exppara.2012.02.005. Epub 2012 Feb 17. Exp Parasitol. 2012. PMID: 22366577
-
Differential expression of microRNAs and tRNA fragments mediate the adaptation of the liver fluke Fasciola gigantica to its intermediate snail and definitive mammalian hosts.Int J Parasitol. 2021 Apr;51(5):405-414. doi: 10.1016/j.ijpara.2020.10.009. Epub 2021 Jan 26. Int J Parasitol. 2021. PMID: 33513403 Review.
-
Fasciola gigantica: studies of the tegument as a basis for the developments of immunodiagnosis and vaccine.Southeast Asian J Trop Med Public Health. 1998 Jun;29(2):387-400. Southeast Asian J Trop Med Public Health. 1998. PMID: 9886134 Review.
Cited by
-
Proteomics coupled with in vitro model to study the early crosstalk occurring between newly excysted juveniles of Fasciola hepatica and host intestinal cells.PLoS Negl Trop Dis. 2022 Oct 12;16(10):e0010811. doi: 10.1371/journal.pntd.0010811. eCollection 2022 Oct. PLoS Negl Trop Dis. 2022. PMID: 36223411 Free PMC article.
-
In silico design of an epitope-based vaccine ensemble for fasliolopsiasis.Front Genet. 2025 Jan 22;15:1451853. doi: 10.3389/fgene.2024.1451853. eCollection 2024. Front Genet. 2025. PMID: 39911308 Free PMC article.
-
Fasciola gigantica Recombinant Abelson Tyrosine Protein Kinase (rFgAbl) Regulates Various Functions of Buffalo Peripheral Blood Mononuclear Cells.Animals (Basel). 2025 Jan 10;15(2):179. doi: 10.3390/ani15020179. Animals (Basel). 2025. PMID: 39858178 Free PMC article.
-
Proteomic analysis of Fasciola gigantica excretory and secretory products (FgESPs) co-immunoprecipitated using a time course of infected buffalo sera.Front Microbiol. 2022 Dec 23;13:1089394. doi: 10.3389/fmicb.2022.1089394. eCollection 2022. Front Microbiol. 2022. PMID: 36620027 Free PMC article.
-
Fasciola gigantica vaccine construct: an in silico approach towards identification and design of a multi-epitope subunit vaccine using calcium binding EF-hand proteins.BMC Immunol. 2023 Jan 5;24(1):1. doi: 10.1186/s12865-022-00535-y. BMC Immunol. 2023. PMID: 36604615 Free PMC article.
References
-
- Spithill TW, Carmona C, Piedrafita D, Smooker PM. Prospects for immunoprophylaxis against Fasciola hepatica (liver fluke) Parasitic Helminths Targets Screens Drugs Vaccines. 2012 doi: 10.1002/9783527652969.ch28. - DOI
-
- Piedrafita D, Spithill T, Smith R, Raadsma H. Improving animal and human health through understanding liver fluke immunology. Parasite Immunol. 2010;32:572–581. - PubMed
-
- Soliman MF. Epidemiological review of human and animal fascioliasis in Egypt. J Infect Dev Countries. 2008;2:182–189. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

