Enhanced wound repair ability of arginine-chitosan nanocomposite membrane through the antimicrobial peptides-loaded polydopamine-modified graphene oxide
- PMID: 34022941
- PMCID: PMC8141257
- DOI: 10.1186/s13036-021-00268-3
Enhanced wound repair ability of arginine-chitosan nanocomposite membrane through the antimicrobial peptides-loaded polydopamine-modified graphene oxide
Abstract
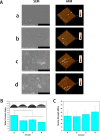
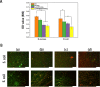
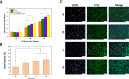
Skin wound healing is a complicated and lengthy process, which is influenced by multiple factors and need a suitable cellular micro-environment. For skin wound, wound dressings remain a cornerstone of dermatologic therapy at present. The dressing material can create an effective protective environment for the wound, and the interactions between the dressing and the wound has a great impact on the wound healing efficiency. An ideal wound dressing materials should have good biocompatibility, moisturizing property, antibacterial property and mechanical strength, and can effectively prevent wound infection and promote wound healing. In this study, in order to design wound dressing materials endowed with excellent antibacterial and tissue repair properties, we attempted to load antimicrobial peptides onto dopmine-modified graphene oxide (PDA@GO) using lysozyme (ly) as a model drug. Then, functionalized GO was used to the surface modification of arginine-modified chitosan (CS-Arg) membrane. To evaluate the potential of the prepared nanocomposite membrane in wound dressing application, the surface morphology, hydrophilic, mechanical properties, antimicrobial activity, and cytocompatibility of the resulting nanocomposite membrane were analyzed. The results revealed that prepared nanocomposite membrane exhibited excellent hydrophilic, mechanical strength and antimicrobial activity, which can effectively promote cell growth and adhesion. In particular, using PDA@GO as drug carrier can effectively maintain the activity of antimicrobial peptides, and can maximize the antibacterial properties of the nanocomposite membrane. Finally, we used rat full-thickness wound models to observe wound healing, and the surface interactions between the prepared nanocomposite membrane and the wound. The results indicated that nanocomposite membrane can obviously accelerated wound closure, and the wounds showed reduced inflammation, improved angiogenesis and accelerated re-epithelialization. Therefore, incorporation of antimicrobial peptides-functionalize graphene oxide (ly-PDA@GO) into CS-Arg membrane was a viable strategy for fabricating excellent wound dressing. Together, this study not only prepared a wound dressing with excellent tissue repair ability, but also provided a novel idea for the development of graphene oxide-based antibacterial dressing.
Keywords: Antimicrobial peptides; Arginine; Chitosan; Graphene oxide; Wound dressing.
Conflict of interest statement
There are no conflicts to declare.
Figures





Similar articles
-
Graphene Oxide/Copper Nanoderivatives-Modified Chitosan/Hyaluronic Acid Dressings for Facilitating Wound Healing in Infected Full-Thickness Skin Defects.Int J Nanomedicine. 2020 Oct 27;15:8231-8247. doi: 10.2147/IJN.S278631. eCollection 2020. Int J Nanomedicine. 2020. PMID: 33149572 Free PMC article.
-
Synthesis of graphene oxide-quaternary ammonium nanocomposite with synergistic antibacterial activity to promote infected wound healing.Burns Trauma. 2018 May 21;6:16. doi: 10.1186/s41038-018-0115-2. eCollection 2018. Burns Trauma. 2018. PMID: 29796394 Free PMC article.
-
Augmented wound healing potential of photosensitive GelMA hydrogel incorporating antimicrobial peptides and MXene nanoparticles.Front Bioeng Biotechnol. 2023 Dec 21;11:1310349. doi: 10.3389/fbioe.2023.1310349. eCollection 2023. Front Bioeng Biotechnol. 2023. PMID: 38179129 Free PMC article.
-
Chitosan-Based Scaffolds Incorporated with Silver Nanoparticles for the Treatment of Infected Wounds.Pharmaceutics. 2024 Feb 26;16(3):327. doi: 10.3390/pharmaceutics16030327. Pharmaceutics. 2024. PMID: 38543221 Free PMC article. Review.
-
Graphene-Based Materials for Inhibition of Wound Infection and Accelerating Wound Healing.Biomed Pharmacother. 2023 Feb;158:114184. doi: 10.1016/j.biopha.2022.114184. Epub 2022 Dec 30. Biomed Pharmacother. 2023. PMID: 36587554 Review.
Cited by
-
Hierarchical double-layer microneedles accomplish multicenter skin regeneration in diabetic full-thickness wounds.J Adv Res. 2024 Dec;66:237-249. doi: 10.1016/j.jare.2024.01.002. Epub 2024 Jan 11. J Adv Res. 2024. PMID: 38218581 Free PMC article.
-
Graphene-based nanomaterials: mechanisms and potentials in the fight against multidrug resistant bacterial infections: a review.RSC Adv. 2025 Jul 28;15(33):26728-26738. doi: 10.1039/d5ra01352f. eCollection 2025 Jul 25. RSC Adv. 2025. PMID: 40727281 Free PMC article. Review.
-
Extraction of Active Compounds from Dioscorea quinqueloba and Their Encapsulation Using Mucin and Chitosan for Application in Cosmetic Formulations.Materials (Basel). 2025 May 8;18(10):2178. doi: 10.3390/ma18102178. Materials (Basel). 2025. PMID: 40428915 Free PMC article.
-
Preparation of metal-organic framework combined with Portulaca oleracea L. extract electrostatically spun nanofiber membranes delayed release wound dressing.RSC Adv. 2023 Jul 19;13(31):21633-21642. doi: 10.1039/d3ra01777j. eCollection 2023 Jul 12. RSC Adv. 2023. PMID: 37476048 Free PMC article.
-
Advances in the application of Mxene nanoparticles in wound healing.J Biol Eng. 2023 Jun 8;17(1):39. doi: 10.1186/s13036-023-00355-7. J Biol Eng. 2023. PMID: 37291625 Free PMC article. Review.
References
-
- Rivero G, Meuter M, Pepe A, Guevara MG, Boccaccini AR, Abraham GA. Nanofibrous membranes as smart wound dressings that release antibiotics when an injury is infected. Colloids Surf a-Physicochemical Eng Aspects. 2020;587:124313. doi: 10.1016/j.colsurfa.2019.124313. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

