Identifying degenerative effects of repetitive head trauma with neuroimaging: a clinically-oriented review
- PMID: 34022959
- PMCID: PMC8141132
- DOI: 10.1186/s40478-021-01197-4
Identifying degenerative effects of repetitive head trauma with neuroimaging: a clinically-oriented review
Abstract
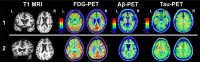
Background and scope of review: Varying severities and frequencies of head trauma may result in dynamic acute and chronic pathophysiologic responses in the brain. Heightened attention to long-term effects of head trauma, particularly repetitive head trauma, has sparked recent efforts to identify neuroimaging biomarkers of underlying disease processes. Imaging modalities like structural magnetic resonance imaging (MRI) and positron emission tomography (PET) are the most clinically applicable given their use in neurodegenerative disease diagnosis and differentiation. In recent years, researchers have targeted repetitive head trauma cohorts in hopes of identifying in vivo biomarkers for underlying biologic changes that might ultimately improve diagnosis of chronic traumatic encephalopathy (CTE) in living persons. These populations most often include collision sport athletes (e.g., American football, boxing) and military veterans with repetitive low-level blast exposure. We provide a clinically-oriented review of neuroimaging data from repetitive head trauma cohorts based on structural MRI, FDG-PET, Aβ-PET, and tau-PET. We supplement the review with two patient reports of neuropathology-confirmed, clinically impaired adults with prior repetitive head trauma who underwent structural MRI, FDG-PET, Aβ-PET, and tau-PET in addition to comprehensive clinical examinations before death.
Review conclusions: Group-level comparisons to controls without known head trauma have revealed inconsistent regional volume differences, with possible propensity for medial temporal, limbic, and subcortical (thalamus, corpus callosum) structures. Greater frequency and severity (i.e., length) of cavum septum pellucidum (CSP) is observed in repetitive head trauma cohorts compared to unexposed controls. It remains unclear whether CSP predicts a particular neurodegenerative process, but CSP presence should increase suspicion that clinical impairment is at least partly attributable to the individual's head trauma exposure (regardless of underlying disease). PET imaging similarly has not revealed a prototypical metabolic or molecular pattern associated with repetitive head trauma or predictive of CTE based on the most widely studied radiotracers. Given the range of clinical syndromes and neurodegenerative pathologies observed in a subset of adults with prior repetitive head trauma, structural MRI and PET imaging may still be useful for differential diagnosis (e.g., assessing suspected Alzheimer's disease).
Keywords: Chronic traumatic encephalopathy; Concussion; Magnetic resonance imaging; Neurodegenerative disease; Neuroimaging; Positron emission tomography; Repetitive head trauma; Traumatic brain injury; Traumatic encephalopathy syndrome.
Conflict of interest statement
BMA and GDR declare that they have no competing interests relevant this review.
Figures

References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

