Sofosbuvir/velpatasvir for 12 vs. 6 weeks for the treatment of recently acquired hepatitis C infection
- PMID: 34023350
- PMCID: PMC9831671
- DOI: 10.1016/j.jhep.2021.04.056
Sofosbuvir/velpatasvir for 12 vs. 6 weeks for the treatment of recently acquired hepatitis C infection
Abstract
Background & aims: Shortened duration therapy for acute and recent HCV infection has been shown to be highly effective in several small non-randomised studies with direct-acting antiviral regimens; however, large randomised studies are lacking.
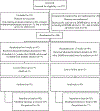
Methods: REACT was an NIH-funded multicentre international, open-label, randomised, phase IV non-inferiority trial examining the efficacy of short course (6-week) vs. standard course (12-week) therapy with sofosbuvir-velpatasvir for recent HCV infection (estimated duration of infection ≤12 months). Randomisation occurred at week 6. The primary endpoint was sustained virological response 12 weeks after treatment end (SVR12) in the intention-to treat (ITT) population. A total of 250 participants were due to be enrolled, but on advice of the data safety and monitoring board the study was halted early.
Results: The primary analysis population consisted of 188 randomised participants at termination of study enrolment; short arm (n = 93), standard arm (n = 95). Ninety-seven percent were male and 69% HIV positive. ITT SVR12 was 76/93, 81.7% (95% CI 72.4-89.0) in the short arm and 86/95, 90.5% (95% CI 82.7-95.6) in the standard arm. The difference between the arms was -8.8 (95% CI -18.6 to 1.0). In modified ITT analysis, wherein non-virological reasons for failure were excluded (death, reinfection, loss to follow-up), SVR12 was 76/85, 89.4% (95% CI 80.8-95.0) in the short arm and 86/88, 97.7% in the standard arm (95% CI 92.0-99.7; difference -8.3%, p = 0.025).
Conclusions: In this randomised study in recent HCV infection, a 6-week course of sofosbuvir-velpatasvir did not meet the criteria for non-inferiority to standard 12-week therapy.
Lay summary: In this randomised trial, 188 people with recently acquired hepatitis C infection were randomly assigned to treatment using either a short 6-week course (93 people) or standard 12-week course (95 people) of the hepatitis C treatment sofosbuvir/velpatasvir. There were 9 cases of relapse after treatment with the short course and 2 following the standard course. A shortened course of 6-week therapy for hepatitis C infection appeared to be less effective than a standard 12-week course in people with recently acquired hepatitis C infection. CLINICALTRIALS.
Gov identifier: NCT02625909.
Keywords: HCV; acute; direct-acting antivirals; recently acquired; short duration; treatment.
Copyright © 2021. Published by Elsevier B.V.
Conflict of interest statement
Conflict of interest GVM: grants from Gilead Sciences and AbbVie Inc, outside the submitted work; SB: grants from Gilead Sciences, outside the submitted work; personal fees for Advisory Boards and lectures/presentations from Gilead Sciences, outside the submitted work; MvDW: grants and personal fees from AbbVie, grants and personal fees from Gilead, grants and personal fees from Johnson & Johnson, grants and personal fees from MSD, grants and personal fees from ViiV, outside the submitted work; JR: personal fees from Gilead Sciences, Janssen, Merck, Theratechnologies and ViiV, outside the submitted work; JF: grants and personal fees from Gilead Sciences, grants and personal fees from AbbVie, personal fees from GSK, personal fees from Roche, grants from Janssen, grants from Eiger, grants and personal fees from Enanta, personal fees from Arubutus, outside the submitted work; AR: Advisory boards: MSD, Gilead Sciences, Travel grants: Gilead Sciences, Pfizer, AbbVie; Research support: Investigator initiated trial grant from Gilead Sciences. All remuneration went to his home institution and not to Dr. Rauch personally; CT: Gilead Advisory board (Remdesivir) 2020; MSD Advisory board (HCV) 2018; educational grants from Gilead and AbbVie (Annual Preceptorship on HCV in PWID), outside the submitted work; JB: grants from NIH, during the conduct of the study; personal fees from AbbVie, grants and personal fees from Gilead, outside the submitted work; AK: grants from PCORI, grants from NIH/NIAID, grants from NIH/NIA, grants from UpToDate, Inc., personal fees from Biomarin, Inc., personal fees for lectures/presentations: CME companies, Clinical Care Options companies, Mentor Planning and Practice Point, personal fees from DKBMed for communications, personal fees for academic work from Geisinger Health Systems and St. Luke’s/Roosevelt, personal fees from Ken Krayesek Law Offices, personal fees from Duke University, outside the submitted work; MH: grants from Gilead Sciences, grants from AbbVie, outside the submitted work; EG: personal fees from Gilead Scientific Advisory Board, personal fees from AbbVie Scientific Advisory Board, personal fees from Janssen Scientific Advisory Board, outside the submitted work; MN: grants, personal fees and non-financial support from AbbVie, grants, personal fees and non-financial support from MSD, grants, personal fees and non-financial support from BMS, grants, personal fees and non-financial support from Gilead Sciences, payment or honoraria: Gilead, AbbVie, BMS and MSD, travel support: Gilead, AbbVie, BMS and MSD, personal fees from MBS DMSB or equivalent, outside the submitted work; PI: grants and personal fees from Gilead Sciences, personal fees from AbbVie, personal fees from ViiV, outside the submitted work; JG: grants and personal fees from AbbVie, grants and personal fees from Gilead Sciences, grants and personal fees from Merck, grants and personal fees from Cepheid, grants from Hologic, grants from Indivior, payment or honoraria: AbbVie, Gilead Sciences and Cepheid, travel support: AbbVie, Gilead Sciences and Cepheid, receipt of testing equipment and cartridges from Cepheid, receipt of testing reagents from Hologic, outside the submitted work; KP: grants from Gilead sciences Australia and ViiV Healthcare Australia, outside the submitted work; GD: grants, personal fees and non-financial support from Gilead Sciences, AbbVie and Merck, grants from Bristol-Myers Squibb, outside the submitted work; DS, MM, TA and PM: nothing to disclose. Please refer to the accompanying ICMJE disclosure forms for further details.
Figures


References
-
- De Rosa FG, Bargiacchi O, Audagnotto S, Garazzino S, Cariti G, Calleri G, et al. Twelve-week treatment of acute hepatitis C virus with pegylated interferon- alpha −2b in injection drug users. Clin Infect Dis. 2007;45(5):583–8. - PubMed
-
- Santantonio T, Fasano M, Sinisi E, Guastadisegni A, Casalino C, Mazzola M, et al. Efficacy of a 24-week course of PEG-interferon alpha-2b monotherapy in patients with acute hepatitis C after failure of spontaneous clearance. J Hepatol. 2005;42(3):329–33. - PubMed
-
- Martinello M, Hellard M, Shaw D, Petoumenos K, Applegate T, Grebely J, et al. Short duration response-guided treatment is effective for most individuals with recent hepatitis C infection: the ATAHC II and DARE-C I studies. Antivir Ther. 2016;21(5):465. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

