Enrichment of NPC1-deficient cells with the lipid LBPA stimulates autophagy, improves lysosomal function, and reduces cholesterol storage
- PMID: 34023384
- PMCID: PMC8294588
- DOI: 10.1016/j.jbc.2021.100813
Enrichment of NPC1-deficient cells with the lipid LBPA stimulates autophagy, improves lysosomal function, and reduces cholesterol storage
Abstract
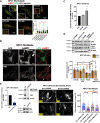
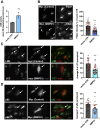
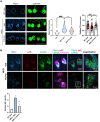
Niemann-Pick C (NPC) is an autosomal recessive disorder characterized by mutations in the NPC1 or NPC2 genes encoding endolysosomal lipid transport proteins, leading to cholesterol accumulation and autophagy dysfunction. We have previously shown that enrichment of NPC1-deficient cells with the anionic lipid lysobisphosphatidic acid (LBPA; also called bis(monoacylglycerol)phosphate) via treatment with its precursor phosphatidylglycerol (PG) results in a dramatic decrease in cholesterol storage. However, the mechanisms underlying this reduction are unknown. In the present study, we showed using biochemical and imaging approaches in both NPC1-deficient cellular models and an NPC1 mouse model that PG incubation/LBPA enrichment significantly improved the compromised autophagic flux associated with NPC1 disease, providing a route for NPC1-independent endolysosomal cholesterol mobilization. PG/LBPA enrichment specifically enhanced the late stages of autophagy, and effects were mediated by activation of the lysosomal enzyme acid sphingomyelinase. PG incubation also led to robust and specific increases in LBPA species with polyunsaturated acyl chains, potentially increasing the propensity for membrane fusion events, which are critical for late-stage autophagy progression. Finally, we demonstrated that PG/LBPA treatment efficiently cleared cholesterol and toxic protein aggregates in Purkinje neurons of the NPC1I1061T mouse model. Collectively, these findings provide a mechanistic basis supporting cellular LBPA as a potential new target for therapeutic intervention in NPC disease.
Keywords: Niemann–Pick type C disease; acid sphingomyelinase; autophagy; cholesterol; lysobisphosphatidic acid.
Copyright © 2021 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest The authors declare that they have no conflicts of interest with the contents of this article.
Figures




Similar articles
-
Molecular determinants of phospholipid treatment to reduce intracellular cholesterol accumulation in NPC1 deficiency.J Biol Chem. 2024 Nov;300(11):107889. doi: 10.1016/j.jbc.2024.107889. Epub 2024 Oct 11. J Biol Chem. 2024. PMID: 39395801 Free PMC article.
-
Intracellular cholesterol trafficking is dependent upon NPC2 interaction with lysobisphosphatidic acid.Elife. 2019 Oct 3;8:e50832. doi: 10.7554/eLife.50832. Elife. 2019. PMID: 31580258 Free PMC article.
-
Beneficial effects of primidone in Niemann-Pick disease type C (NPC)-model cells and mice: Reduction of unesterified cholesterol levels in cells and extension of lifespan in mice.Eur J Pharmacol. 2021 Apr 5;896:173907. doi: 10.1016/j.ejphar.2021.173907. Epub 2021 Jan 24. Eur J Pharmacol. 2021. PMID: 33503462
-
Niemann-Pick type C disease: molecular mechanisms and potential therapeutic approaches.J Neurochem. 2011 Mar;116(5):789-95. doi: 10.1111/j.1471-4159.2010.06976.x. Epub 2011 Jan 7. J Neurochem. 2011. PMID: 20807315 Free PMC article. Review.
-
Endo-lysosomal dysfunction and neuronal-glial crosstalk in Niemann-Pick type C disease.Philos Trans R Soc Lond B Biol Sci. 2024 Apr 8;379(1899):20220388. doi: 10.1098/rstb.2022.0388. Epub 2024 Feb 19. Philos Trans R Soc Lond B Biol Sci. 2024. PMID: 38368932 Free PMC article. Review.
Cited by
-
Did mitophagy follow the origin of mitochondria?Autophagy. 2024 May;20(5):985-993. doi: 10.1080/15548627.2024.2307215. Epub 2024 Feb 15. Autophagy. 2024. PMID: 38361280 Free PMC article. Review.
-
NPC1 promotes the progression of hepatocellular carcinoma by mediating the accumulation of neutrophils into the tumor microenvironment.FEBS Open Bio. 2025 Apr;15(4):661-673. doi: 10.1002/2211-5463.13951. Epub 2024 Dec 20. FEBS Open Bio. 2025. PMID: 39707615 Free PMC article.
-
Structural Predictions of the SNX-RGS Proteins Suggest They Belong to a New Class of Lipid Transfer Proteins.Front Cell Dev Biol. 2022 Feb 3;10:826688. doi: 10.3389/fcell.2022.826688. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35223850 Free PMC article.
-
Cholesterol content regulates silica-induced lysosomal membrane permeability.Front Toxicol. 2023 Feb 13;5:1112822. doi: 10.3389/ftox.2023.1112822. eCollection 2023. Front Toxicol. 2023. PMID: 36860548 Free PMC article.
-
Association Study Between Polymorphic Loci in Cholesterol Metabolism Pathway and Gallstone in the Tibetan Population.Front Genet. 2022 May 16;13:902553. doi: 10.3389/fgene.2022.902553. eCollection 2022. Front Genet. 2022. PMID: 35651949 Free PMC article.
References
-
- Carstea E.D., Morris J.A., Coleman K.G., Loftus S.K., Zhang D., Cummings C., Gu J., Rosenfeld M.A., Pavan W.J., Krizman D.B., Nagle J., Polymeropoulos M.H., Sturley S.L., Ioannou Y.A., Higgins M.E. Niemann-Pick C1 disease gene: Homology to mediators of cholesterol homeostasis. Science. 1997;277:228–231. - PubMed
-
- Naureckiene S., Sleat D.E., Lackland H., Fensom A., Vanier M.T., Wattiaux R., Jadot M., Lobel P. Identification of HE1 as the second gene of Niemann-Pick C disease. Science. 2000;290:2298–2301. - PubMed
-
- Vanier M.T. Complex lipid trafficking in Niemann-Pick disease type C. J. Inherit. Metab. Dis. 2015;38:187–199. - PubMed
-
- Mobius W., van Donselaar E., Ohno-Iwashita Y., Shimada Y., Heijnen H.F., Slot J.W., Geuze H.J. Recycling compartments and the internal vesicles of multivesicular bodies harbor most of the cholesterol found in the endocytic pathway. Traffic. 2003;4:222–231. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

