Drug combination therapy for emerging viral diseases
- PMID: 34023496
- PMCID: PMC8139175
- DOI: 10.1016/j.drudis.2021.05.008
Drug combination therapy for emerging viral diseases
Abstract
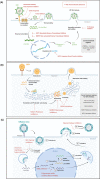
Effective therapeutics to combat emerging viral infections are an unmet need. Historically, treatments for chronic viral infections with single drugs have not been successful, as exemplified by human immunodeficiency virus (HIV) and hepatitis C virus (HCV) infections. Combination therapy for these diseases has led to improved clinical outcomes with dramatic reductions in viral load, morbidity, and mortality. Drug combinations can enhance therapeutic efficacy through additive, and ideally synergistic, effects for emerging and re-emerging viruses, such as influenza, severe acute respiratory syndrome-coronavirus (SARS-CoV), Middle East respiratory syndrome (MERS)-CoV, Ebola, Zika, and SARS-coronavirus 2 (CoV-2). Although novel drug development through traditional pipelines remains a priority, in the interim, effective synergistic drug candidates could be rapidly identified by drug-repurposing screens, facilitating accelerated paths to clinical testing and potential emergency use authorizations.
Keywords: COVID-19; Drug combination therapy; Emerging viral diseases; SARS-CoV-2.
Copyright © 2021 The Authors. Published by Elsevier Ltd.. All rights reserved.
Figures

Similar articles
-
Old Weapon for New Enemy: Drug Repurposing for Treatment of Newly Emerging Viral Diseases.Virol Sin. 2020 Jun;35(3):253-255. doi: 10.1007/s12250-020-00204-7. Epub 2020 Feb 11. Virol Sin. 2020. PMID: 32048130 Free PMC article. No abstract available.
-
Compounds with Therapeutic Potential against Novel Respiratory 2019 Coronavirus.Antimicrob Agents Chemother. 2020 Apr 21;64(5):e00399-20. doi: 10.1128/AAC.00399-20. Print 2020 Apr 21. Antimicrob Agents Chemother. 2020. PMID: 32152082 Free PMC article. Review.
-
Human monoclonal antibodies as candidate therapeutics against emerging viruses.Front Med. 2017 Dec;11(4):462-470. doi: 10.1007/s11684-017-0596-6. Epub 2017 Nov 20. Front Med. 2017. PMID: 29159596 Free PMC article. Review.
-
Development of small-molecule viral inhibitors targeting various stages of the life cycle of emerging and re-emerging viruses.Front Med. 2017 Dec;11(4):449-461. doi: 10.1007/s11684-017-0589-5. Epub 2017 Nov 23. Front Med. 2017. PMID: 29170916 Free PMC article. Review.
-
Intervention strategies for emerging viruses: use of antivirals.Curr Opin Virol. 2013 Apr;3(2):217-24. doi: 10.1016/j.coviro.2013.03.001. Epub 2013 Apr 4. Curr Opin Virol. 2013. PMID: 23562753 Free PMC article. Review.
Cited by
-
Assessment of Favipiravir and Remdesivir in Combination for SARS-CoV-2 Infection in Syrian Golden Hamsters.Viruses. 2024 Nov 27;16(12):1838. doi: 10.3390/v16121838. Viruses. 2024. PMID: 39772148 Free PMC article.
-
SARS-CoV-2 Main Protease Drug Design, Assay Development, and Drug Resistance Studies.Acc Chem Res. 2023 Jan 17;56(2):157-168. doi: 10.1021/acs.accounts.2c00735. Epub 2022 Dec 29. Acc Chem Res. 2023. PMID: 36580641 Free PMC article.
-
The triple combination of Remdesivir (GS-441524), Molnupiravir and Ribavirin is highly efficient in inhibiting coronavirus replication in human nasal airway epithelial cell cultures and in a hamster infection model.bioRxiv [Preprint]. 2024 May 15:2024.05.14.594200. doi: 10.1101/2024.05.14.594200. bioRxiv. 2024. Update in: Antiviral Res. 2024 Nov;231:105994. doi: 10.1016/j.antiviral.2024.105994. PMID: 38798406 Free PMC article. Updated. Preprint.
-
Drupacine as a potent SARS-CoV-2 replication inhibitor in vitro.Biosaf Health. 2024 Sep 3;6(5):270-278. doi: 10.1016/j.bsheal.2024.09.001. eCollection 2024 Oct. Biosaf Health. 2024. PMID: 40078736 Free PMC article.
-
Inhaled Dry Powder of Antiviral Agents: A Promising Approach to Treating Respiratory Viral Pathogens.Viruses. 2025 Feb 12;17(2):252. doi: 10.3390/v17020252. Viruses. 2025. PMID: 40007007 Free PMC article. Review.
References
-
- 1918 Pandemic. Centers for Disease Control and Prevention Website. www.cdc.gov/flu/pandemic-resources/1918-pandemic-h1n1.html. Updated March 20 2019 [Accessed May 14, 2021]
-
- Severe Acute Respiratory Syndrome. Centers for Disease Control and Prevention Website. www.cdc.gov/sars/about/fs-sars.html/. Updated December 6, 2017. [Accessed May 14, 2021]
-
- Middle East Respiratory Syndrome Virus. WHO Website. www.who.int/news-room/fact-sheets/detail/middle-east-respiratory-syndrom.... Updated March 11, 2019. [Accessed May 14, 2021]
-
- Ebola Virus Disease. WHO Website. www.who.int/en/news-room/fact-sheets/detail/ebola-virus-disease. Updated February 23, 2021. [Accessed May 14, 2021]
-
- Ebola Virus Disease. Centers for Disease Control and Prevention Website. www.cdc.gov/vhf/ebola/clinicians/vaccine/index.html. Updated February 25, 2021. [Accessed May 14, 2021]
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

