Exploring the reporting standards of RCTs involving invasive procedures for assisted vaginal birth: A systematic review
- PMID: 34023718
- PMCID: PMC8250286
- DOI: 10.1016/j.ejogrb.2021.05.026
Exploring the reporting standards of RCTs involving invasive procedures for assisted vaginal birth: A systematic review
Abstract
Objective: Assisted vaginal birth (AVB) is a complex intervention involving medical devices, comprising multiple components. This complexity creates difficulties when designing and conducting randomised controlled trials (RCTs), in terms of describing, standardising and monitoring the intervention, and accounting for differing clinician expertise. This review examines the reporting standards of complex interventions involving a medical device, in the context of AVB RCTs.
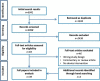
Study design: Searches were undertaken from the start of indexing to March 2021, and limited to RCTs, feasibility and pilot studies including at least one device for AVB. RCTs were selected if they included participants having an AVB with any device, with or without a comparator group. Reporting details were assessed according to the Consolidating Standards of Reporting Trials extension for non-pharmacological treatments (CONSORT-NPT), focusing on intervention descriptions, standardization, adherence and clinician expertise. Screening of abstracts, full-text articles and data extraction was performed by two independent reviewers.
Results: Of 4098 abstracts and 83 full-text articles, 39 papers were included, investigating 80 interventions. Twenty-seven different named devices were identified. Intervention descriptions were provided in 20 (55%) papers with varying levels of detail and with only one covering the entire procedure. Standardization of interventions was mentioned in 25 papers (64%). Only eight (21%) papers reported any form of adherence to the intended procedure. Some data regarding expertise were reported in 25 (64%) papers.
Conclusions: Despite some compliance with reporting standards, there is a lack of detail regarding intervention description, standardization, adherence and expertise in RCTs of AVB. This creates difficulties in understanding how intervention delivery was intended and what actually occurred. Clearer guidelines for the reporting of invasive procedures and devices are required.
Keywords: Assisted vaginal birth; Complex interventions; Forceps; Odon Device; Reporting standards; Ventouse.
Copyright © 2021 The Author(s). Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest EJH, SR, JFC, TJD are employees of North Bristol NHS Trust, which receives funding from PROMPT Maternity Foundation (PMF) to pay part of their salaries. PMF has received funds from Becton Dickinson, manufacturer of the Odon Device to run pre-clinical simulation studies on the device. EL is an employee of the University of Bristol, which receives funding from PMF to pay part of EL’s salary. NSB is an MRC Clinician Scientist.
Figures
Similar articles
-
Interventions for promoting habitual exercise in people living with and beyond cancer.Cochrane Database Syst Rev. 2018 Sep 19;9(9):CD010192. doi: 10.1002/14651858.CD010192.pub3. Cochrane Database Syst Rev. 2018. PMID: 30229557 Free PMC article.
-
A rapid and systematic review of the clinical effectiveness and cost-effectiveness of topotecan for ovarian cancer.Health Technol Assess. 2001;5(28):1-110. doi: 10.3310/hta5280. Health Technol Assess. 2001. PMID: 11701100
-
Exploring the Reporting Standards of Randomised Controlled Trials Involving Endovascular Interventions for Peripheral Arterial Disease: A Systematic Review.Eur J Vasc Endovasc Surg. 2024 Jan;67(1):155-164. doi: 10.1016/j.ejvs.2023.08.066. Epub 2023 Sep 9. Eur J Vasc Endovasc Surg. 2024. PMID: 37678660
-
Interventions for infantile haemangiomas of the skin.Cochrane Database Syst Rev. 2018 Apr 18;4(4):CD006545. doi: 10.1002/14651858.CD006545.pub3. Cochrane Database Syst Rev. 2018. PMID: 29667726 Free PMC article.
-
Systematic review of intervention design and delivery in pragmatic and explanatory surgical randomized clinical trials.Br J Surg. 2015 Aug;102(9):1037-47. doi: 10.1002/bjs.9808. Epub 2015 Jun 3. Br J Surg. 2015. PMID: 26041565
Cited by
-
Engaging women to set the research agenda for assisted vaginal birth.Health Expect. 2024 Jun;27(3):e14054. doi: 10.1111/hex.14054. Health Expect. 2024. PMID: 38877659 Free PMC article.
-
A research agenda to improve incidence and outcomes of assisted vaginal birth.Bull World Health Organ. 2023 Nov 1;101(11):723-729. doi: 10.2471/BLT.23.290140. Epub 2023 Oct 4. Bull World Health Organ. 2023. PMID: 37961052 Free PMC article.
References
-
- Hirshberg A., Srinivas S.K. Role of operative vaginal deliveries in prevention of cesarean deliveries. Clin Obstet Gynecol. 2015;58(2):256–262. - PubMed
-
- Tarricone R., Torbica A., Ferré F., Drummond M. Generating appropriate clinical data for value assessment of medical devices: what role does regulation play? Expert Rev Pharmacoecon Outcomes Res. 2014;14(October (5)):707–718. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources