Hypoxia-Inducible Factor 1 Alpha Is Dispensable for Host Defense of Group B Streptococcus Colonization and Infection
- PMID: 34023827
- PMCID: PMC8613573
- DOI: 10.1159/000515739
Hypoxia-Inducible Factor 1 Alpha Is Dispensable for Host Defense of Group B Streptococcus Colonization and Infection
Abstract
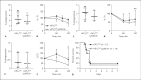
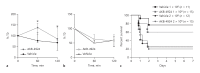
Group B Streptococcus (GBS) is a leading cause of neonatal morbidity and mortality, and the primary source of exposure is the maternal vagina. Intrapartum antibiotic prophylaxis for GBS-positive mothers has reduced the incidence of GBS early-onset disease, however, potential long-lasting influence of an antibiotic-altered neonatal microbiota, and the frequent clinical sequelae in survivors of invasive GBS infection, compels alternative treatment options for GBS. Here, we examined the role of transcription factor hypoxia-inducible factor 1 alpha (HIF-1α), widely recognized as a regulator of immune activation during infection, in the host response to GBS. Given the importance of endogenous HIF-1α for innate immune defense, and the potential utility of HIF-1α stabilization in promoting bacterial clearance, we hypothesized that HIF-1α could play an important role in coordinating host responses to GBS in colonization and systemic disease. Counter to our hypothesis, we found that GBS infection did not induce HIF-1α expression in vaginal epithelial cells or murine macrophages, nor did HIF-1α deficiency alter GBS colonization or pathogenesis in vivo. Furthermore, pharmacological enhancement of HIF-1α did not improve control of GBS in pathogenesis and colonization models, while displaying inhibitory effects in vaginal epithelial cytokines and immune cell killing in vitro. Taken together, we conclude that HIF-1α is not a prominent aspect of the host response to GBS colonization or invasive disease, and its pharmacological modulation is unlikely to provide significant benefit against this important neonatal pathogen.
Keywords: Group B streptococcus; Hypoxia-inducible factor 1 alpha; Innate immunity; Macrophage; Vaginal colonization.
© 2021 The Author(s). Published by S. Karger AG, Basel.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Figures


References
-
- Libster R, Edwards KM, Levent F, Edwards MS, Rench MA, Castagnini LA, et al. Long-term outcomes of group B streptococcal meningitis. Pediatrics. 2012;130((1)):e8–15. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

