Plastin 3 in health and disease: a matter of balance
- PMID: 34023917
- PMCID: PMC8257523
- DOI: 10.1007/s00018-021-03843-5
Plastin 3 in health and disease: a matter of balance
Abstract
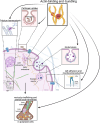
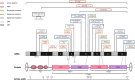
For a long time, PLS3 (plastin 3, also known as T-plastin or fimbrin) has been considered a rather inconspicuous protein, involved in F-actin-binding and -bundling. However, in recent years, a plethora of discoveries have turned PLS3 into a highly interesting protein involved in many cellular processes, signaling pathways, and diseases. PLS3 is localized on the X-chromosome, but shows sex-specific, inter-individual and tissue-specific expression variability pointing towards skewed X-inactivation. PLS3 is expressed in all solid tissues but usually not in hematopoietic cells. When escaping X-inactivation, PLS3 triggers a plethora of different types of cancers. Elevated PLS3 levels are considered a prognostic biomarker for cancer and refractory response to therapies. When it is knocked out or mutated in humans and mice, it causes osteoporosis with bone fractures; it is the only protein involved in actin dynamics responsible for osteoporosis. Instead, when PLS3 is upregulated, it acts as a highly protective SMN-independent modifier in spinal muscular atrophy (SMA). Here, it seems to counteract reduced F-actin levels by restoring impaired endocytosis and disturbed calcium homeostasis caused by reduced SMN levels. In contrast, an upregulation of PLS3 on wild-type level might cause osteoarthritis. This emphasizes that the amount of PLS3 in our cells must be precisely balanced; both too much and too little can be detrimental. Actin-dynamics, regulated by PLS3 among others, are crucial in a lot of cellular processes including endocytosis, cell migration, axonal growth, neurotransmission, translation, and others. Also, PLS3 levels influence the infection with different bacteria, mycosis, and other pathogens.
Keywords: Amyotrophic lateral sclerosis; Ataxia; Colorectal cancer; Cutaneous T-cell lymphomas; Osteoclasts.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
- Wi 945/17-1/Deutsche Forschungsgemeinschaft
- FOR2722, project ID 407176282/Deutsche Forschungsgemeinschaft
- SFB 1451, project-ID 431549029 - A01/Deutsche Forschungsgemeinschaft
- GRK1960, project ID 233886668/Deutsche Forschungsgemeinschaft
- Marie Skłodowska-Curie grant agreement No 956185 (SMABEYOND)/H2020 European Research Council
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

