Glycosylation is a key in SARS-CoV-2 infection
- PMID: 34023935
- PMCID: PMC8140746
- DOI: 10.1007/s00109-021-02092-0
Glycosylation is a key in SARS-CoV-2 infection
Abstract
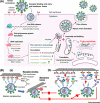
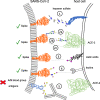
SARS-CoV-2 causes the respiratory syndrome COVID-19 and is responsible for the current pandemic. The S protein of SARS-CoV-2-mediating virus binding to target cells and subsequent viral uptake is extensively glycosylated. Here we focus on how glycosylation of both SARS-CoV-2 and target cells crucially impacts SARS-CoV-2 infection at different levels: (1) virus binding and entry to host cells, with glycosaminoglycans of host cells acting as a necessary co-factor for SARS-CoV-2 infection by interacting with the receptor-binding domain of the SARS-CoV-2 spike glycoprotein, (2) innate and adaptive immune response where glycosylation plays both a protective role and contributes to immune evasion by masking of viral polypeptide epitopes and may add to the cytokine cascade via non-fucosylated IgG, and (3) therapy and vaccination where a monoclonal antibody-neutralizing SARS-CoV-2 was shown to interact also with a distinct glycan epitope on the SARS-CoV-2 spike protein. These evidences highlight the importance of ensuring that glycans are considered when tackling this disease, particularly in the development of vaccines, therapeutic strategies and serological testing.
Keywords: Blood group antigen; COVID-19; Glycosylation; Infection; SARS-CoV-2; Spike protein.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

