Serum IgG levels in children 6 months after SARS-CoV-2 infection and comparison with adults
- PMID: 34023936
- PMCID: PMC8140562
- DOI: 10.1007/s00431-021-04124-w
Serum IgG levels in children 6 months after SARS-CoV-2 infection and comparison with adults
Abstract
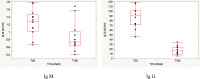
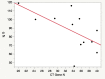
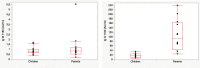
Since the outbreak of SARS-CoV-2 among the population has occurred quite recently, there is a lack of evidence on the long-term duration of antibody response, especially in children. It is therefore crucial to clarify this aspect, considering its implications in the development of successful surveillance strategies, therapies, and vaccinations. The aim of this study was to assess the antibody response in a children group after SARS-CoV-2 infection, and to compare it with that of their parents affected by SARS-CoV-2 infection. We enrolled 12 children and their parents, both groups being affected by COVID-19 in April 2020. In the children's group, we collected real-time RT-PCR cycle threshold (Ct) values and gene characterization of first nasal-throat swab at the time of diagnosis (T0); 30 days after the diagnosis (T30), we performed blood tests to detect anti-SARS-CoV-2 IgM and IgG. Finally, 180 days after the diagnosis (T180), we measured anti-SARS-CoV-2 IgG in both children and parents. In children, antibody levels declined significantly at 180 days (T180) after first measurement (T30). There were no significant differences in IgG level related to age, sex, and clinical manifestations. We found a significant correlation between IgG titers at T30 and Ct value of gene N. Children showed a lower level of antibodies against SARS-CoV-2 at T180 compared to their parents.Conclusion: Antibody responses in children waned 180 days after SARS-CoV-2 infection, and at the same time, their parents showed a different antibody response to the virus. These results highlight that serological tests should be used with caution in surveillance strategies among the general population. What is known: • Currently is not known how long antibody response will be maintained or if it protects from reinfection. • Recent reports in adults suggest that antibodies to SARS-CoV-2 declined several months after infection, but data are missing in pediatric age. What is new: • We showed that antibody responses to SARS-CoV-2 wane several months after infection also in children with quantitative differences in antibody levels between children and adults. • In this context, serological tests should be used with caution in surveillance strategies.
Keywords: Antibody response; COVID-19; Children; Immunity.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
References
-
- World Health Organization Press Conference. The World Health Organization (WHO) has officially named the disease caused by the novel coronavirus as COVID-19. Available online: https://www.who.int/ emergencies/diseases/novel-coronavirus-2019.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

