Arrhythmia Mechanism and Dynamics in a Humanized Mouse Model of Inherited Cardiomyopathy Caused by Phospholamban R14del Mutation
- PMID: 34024116
- PMCID: PMC8456417
- DOI: 10.1161/CIRCULATIONAHA.119.043502
Arrhythmia Mechanism and Dynamics in a Humanized Mouse Model of Inherited Cardiomyopathy Caused by Phospholamban R14del Mutation
Abstract
Background: Arginine (Arg) 14 deletion (R14del) in the calcium regulatory protein phospholamban (hPLNR14del) has been identified as a disease-causing mutation in patients with an inherited cardiomyopathy. Mechanisms underlying the early arrhythmogenic phenotype that predisposes carriers of this mutation to sudden death with no apparent structural remodeling remain unclear.
Methods: To address this, we performed high spatiotemporal resolution optical mapping of intact hearts from adult knock-in mice harboring the human PLNWT (wildtype [WT], n=12) or the heterozygous human PLNR14del mutation (R14del, n=12) before and after ex vivo challenge with isoproterenol and rapid pacing.
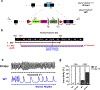
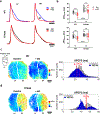
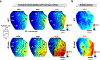
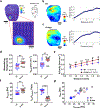
Results: Adverse electrophysiological remodeling was evident in the absence of significant structural or hemodynamic changes. R14del hearts exhibited increased arrhythmia susceptibility compared with wildtype. Underlying this susceptibility was preferential right ventricular action potential prolongation that was unresponsive to β-adrenergic stimulation. A steep repolarization gradient at the left ventricular/right ventricular interface provided the substrate for interventricular activation delays and ultimately local conduction block during rapid pacing. This was followed by the initiation of macroreentrant circuits supporting the onset of ventricular tachycardia. Once sustained, these circuits evolved into high-frequency rotors, which in their majority were pinned to the right ventricle. These rotors exhibited unique spatiotemporal dynamics that promoted their increased stability in R14del compared with wildtype hearts.
Conclusions: Our findings highlight the crucial role of primary electric remodeling caused by the hPLNR14del mutation. These inherently arrhythmogenic features form the substrate for adrenergic-mediated VT at early stages of PLNR14del induced cardiomyopathy.
Keywords: arrhythmia; arrhythmogenic cardiomyopathy; dilated cardiomyopathy; dynamics; phospholamban; spiral wave reentry; sudden death; ventricular tachycardia.
Figures



Similar articles
-
Myofilament Alterations Associated with Human R14del-Phospholamban Cardiomyopathy.Int J Mol Sci. 2023 Jan 31;24(3):2675. doi: 10.3390/ijms24032675. Int J Mol Sci. 2023. PMID: 36768995 Free PMC article.
-
Gene editing reverses arrhythmia susceptibility in humanized PLN-R14del mice: modelling a European cardiomyopathy with global impact.Cardiovasc Res. 2022 Dec 9;118(15):3140-3150. doi: 10.1093/cvr/cvac021. Cardiovasc Res. 2022. PMID: 35191471 Free PMC article.
-
Unfolded Protein Response as a Compensatory Mechanism and Potential Therapeutic Target in PLN R14del Cardiomyopathy.Circulation. 2021 Aug 3;144(5):382-392. doi: 10.1161/CIRCULATIONAHA.120.049844. Epub 2021 Apr 30. Circulation. 2021. PMID: 33928785 Free PMC article.
-
Prevalence and cardiac phenotype of patients with a phospholamban mutation.Neth Heart J. 2019 Feb;27(2):64-69. doi: 10.1007/s12471-018-1211-4. Neth Heart J. 2019. PMID: 30547415 Free PMC article. Review.
-
Phospholamban R14del disease: The past, the present and the future.Front Cardiovasc Med. 2023 Apr 18;10:1162205. doi: 10.3389/fcvm.2023.1162205. eCollection 2023. Front Cardiovasc Med. 2023. PMID: 37144056 Free PMC article. Review.
Cited by
-
Exercise Causes Arrhythmogenic Remodeling of Intracellular Calcium Dynamics in Plakophilin-2-Deficient Hearts.Circulation. 2022 May 10;145(19):1480-1496. doi: 10.1161/CIRCULATIONAHA.121.057757. Epub 2022 May 1. Circulation. 2022. PMID: 35491884 Free PMC article.
-
Genetic landscape of phospholamban cardiomyopathies.Front Cell Dev Biol. 2025 Jun 10;13:1626242. doi: 10.3389/fcell.2025.1626242. eCollection 2025. Front Cell Dev Biol. 2025. PMID: 40556736 Free PMC article. Review.
-
Empagliflozin rescues pro-arrhythmic and Ca2+ homeostatic effects of transverse aortic constriction in intact murine hearts.Sci Rep. 2024 Jul 8;14(1):15683. doi: 10.1038/s41598-024-66098-7. Sci Rep. 2024. PMID: 38977794 Free PMC article.
-
Myofilament Alterations Associated with Human R14del-Phospholamban Cardiomyopathy.Int J Mol Sci. 2023 Jan 31;24(3):2675. doi: 10.3390/ijms24032675. Int J Mol Sci. 2023. PMID: 36768995 Free PMC article.
-
Gene editing reverses arrhythmia susceptibility in humanized PLN-R14del mice: modelling a European cardiomyopathy with global impact.Cardiovasc Res. 2022 Dec 9;118(15):3140-3150. doi: 10.1093/cvr/cvac021. Cardiovasc Res. 2022. PMID: 35191471 Free PMC article.
References
-
- Braunwald E. Cardiomyopathies: An Overview. Circ Res. 2017;121:711–721. - PubMed
-
- Haghighi K, Kolokathis F, Gramolini AO, Waggoner JR, Pater L, Lynch RA, Fan GC, Tsiapras D, Parekh RR, Dorn GW 2nd, MacLennan DH, Kremastinos DT and Kranias EG. A mutation in the human phospholamban gene, deleting arginine 14, results in lethal, hereditary cardiomyopathy. Proc Natl Acad Sci U S A. 2006;103:1388–93. - PMC - PubMed
-
- Bers DM. Calcium cycling and signaling in cardiac myocytes. Annu Rev Physiol. 2008;70:23–49. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

