FLT3 tyrosine kinase inhibitors synergize with BCL-2 inhibition to eliminate FLT3/ITD acute leukemia cells through BIM activation
- PMID: 34024909
- PMCID: PMC8141515
- DOI: 10.1038/s41392-021-00578-4
FLT3 tyrosine kinase inhibitors synergize with BCL-2 inhibition to eliminate FLT3/ITD acute leukemia cells through BIM activation
Abstract
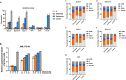
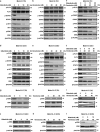
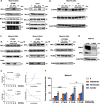
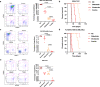
Tyrosine kinase inhibitors (TKIs) targeting FLT3 have shown activity but when used alone have achieved limited success in clinical trials, suggesting the need for combination with other drugs. We investigated the combination of FLT3 TKIs (Gilteritinib or Sorafenib), with Venetoclax, a BCL-2 selective inhibitor (BCL-2i), on FLT3/ITD leukemia cells. The combination of a FLT3 TKI and a BCL-2i synergistically reduced cell proliferation and enhanced apoptosis/cell death in FLT3/ITD cell lines and primary AML samples. Venetoclax also re-sensitized FLT3 TKI-resistant cells to Gilteritinib or Sorafenib treatment, mediated through MAPK pathway inhibition. Gilteritinib treatment alone dissociated BIM from MCL-1 but increased the binding of BIM to BCL-2. Venetoclax treatment enhanced the binding of BIM to MCL-1 but dissociated BIM from BCL-2. Treatment with the drugs together resulted in dissociation of BIM from both BCL-2 and MCL-1, with an increased binding of BIM to the cell death mediator BAX, leading to increased apoptosis. These findings suggest that Venetoclax mitigates the unintended pro-survival effects of FLT3 TKI mainly through the dissociation of BIM and BCL-2 and also decreased BIM expression. This study provides evidence that the addition of BCL-2i enhances the effect of FLT3 TKI therapy in FLT3/ITD AML treatment.
Conflict of interest statement
The authors declare no conflicts of interest. D.S. does serve on the SAB of InSilico Medicine and receives research support and serves as a consultant for an unrelated project from PHarosI&BT co, Ltd. M.L. has received honoraria from Daiichi Sankyo, Novartis, and Agios; has served in a consulting or advisory role for Daiichi Sankyo, Novartis, and Agios; and has received research funding from Astellas and Novartis.
Figures



References
-
- Kottaridis PD, et al. The presence of a FLT3 internal tandem duplication in patients with acute myeloid leukemia (AML) adds important prognostic information to cytogenetic risk group and response to the first cycle of chemotherapy: analysis of 854 patients from the United Kingdom Medical Research Council AML 10 and 12 trials. Blood. 2001;98:1752–1759. doi: 10.1182/blood.V98.6.1752. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

