Alleviation of acute pancreatitis-associated lung injury by inhibiting the p38 mitogen-activated protein kinase pathway in pulmonary microvascular endothelial cells
- PMID: 34025070
- PMCID: PMC8117735
- DOI: 10.3748/wjg.v27.i18.2141
Alleviation of acute pancreatitis-associated lung injury by inhibiting the p38 mitogen-activated protein kinase pathway in pulmonary microvascular endothelial cells
Abstract
Background: Previous reports have suggested that the p38 mitogen-activated protein kinase signaling pathway is involved in the development of severe acute pancreatitis (SAP)-related acute lung injury (ALI). Inhibition of p38 by SB203580 blocked the inflammatory responses in SAP-ALI. However, the precise mechanism associated with p38 is unclear, particularly in pulmonary microvascular endothelial cell (PMVEC) injury.
Aim: To determine its role in the tumor necrosis factor-alpha (TNF-α)-induced inflammation and apoptosis of PMVECs in vitro. We then conducted in vivo experiments to confirm the effect of SB203580-mediated p38 inhibition on SAP-ALI.
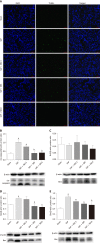
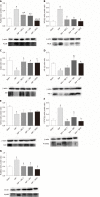
Methods: In vitro, PMVEC were transfected with mitogen-activated protein kinase kinase 6 (Glu), which constitutively activates p38, and then stimulated with TNF-α. Flow cytometry and western blotting were performed to detect the cell apoptosis and inflammatory cytokine levels, respectively. In vivo, SAP-ALI was induced by 5% sodium taurocholate and three different doses of SB203580 (2.5, 5.0 or 10.0 mg/kg) were intraperitoneally injected prior to SAP induction. SAP-ALI was assessed by performing pulmonary histopathology assays, measuring myeloperoxidase activity, conducting arterial blood gas analyses and measuring TNF-α, interleukin (IL)-1β and IL-6 levels. Lung microvascular permeability was measured by determining bronchoalveolar lavage fluid protein concentration, Evans blue extravasation and ultrastructural changes in PMVECs. The apoptotic death of pulmonary cells was confirmed by performing a terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling analysis and examining the Bcl2, Bax, Bim and cle-caspase3 levels. The proteins levels of P-p38, NFκB, IκB, P-signal transducer and activator of transcription-3, nuclear factor erythroid 2-related factor 2, HO-1 and Myd88 were detected in the lungs to further evaluate the potential mechanism underlying the protective effect of SB203580.
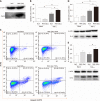
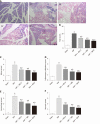
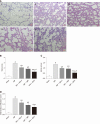
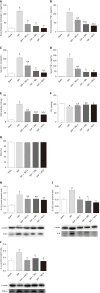
Results: In vitro, mitogen-activated protein kinase (Glu) transfection resulted in higher apoptotic rates and cytokine (IL-1β and IL-6) levels in TNF-α-treated PMVECs. In vivo, SB2035080 attenuated lung histopathological injury, decreased inflammatory activity (TNF-α, IL-1β, IL-6 and myeloperoxidase) and preserved pulmonary function. Furthermore, SB203580 significantly reversed changes in the bronchoalveolar lavage fluid protein concentration, Evans blue accumulation, terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling-positive cell numbers, apoptosis-related proteins (cle-caspase3, Bim and Bax) and endothelial microstructure. Moreover, SB203580 significantly reduced the pulmonary P-p38, NFκB, P-signal transducer and activator of transcription-3 and Myd88 levels but increased the IκB and HO-1 levels.
Conclusion: p38 inhibition may protect against SAP-ALI by alleviating inflammation and the apoptotic death of PMVECs.
Keywords: Acute lung injury; Acute pancreatitis; Apoptosis; P38; Pulmonary microvascular endothelial cells; SB203580.
©The Author(s) 2021. Published by Baishideng Publishing Group Inc. All rights reserved.
Conflict of interest statement
Conflict-of-interest statement: The authors declare that they have no conflict of interest.
Figures

Similar articles
-
Inhibition of SOCs Attenuates Acute Lung Injury Induced by Severe Acute Pancreatitis in Rats and PMVECs Injury Induced by Lipopolysaccharide.Inflammation. 2016 Jun;39(3):1049-58. doi: 10.1007/s10753-016-0335-1. Inflammation. 2016. PMID: 27025854
-
MicroRNA-339-3p alleviates inflammation and edema and suppresses pulmonary microvascular endothelial cell apoptosis in mice with severe acute pancreatitis-associated acute lung injury by regulating Anxa3 via the Akt/mTOR signaling pathway.J Cell Biochem. 2018 Aug;119(8):6704-6714. doi: 10.1002/jcb.26859. Epub 2018 Apr 25. J Cell Biochem. 2018. Retraction in: J Cell Biochem. 2021 Nov;122 Suppl 1:S107. doi: 10.1002/jcb.30087. PMID: 29693276 Retracted.
-
microRNA-542-5p protects against acute lung injury in mice with severe acute pancreatitis by suppressing the mitogen-activated protein kinase signaling pathway through the negative regulation of P21-activated kinase 1.J Cell Biochem. 2019 Jan;120(1):290-304. doi: 10.1002/jcb.27356. Epub 2018 Sep 14. J Cell Biochem. 2019. Retraction in: J Cell Biochem. 2021 Nov;122 Suppl 1:S117. doi: 10.1002/jcb.30097. PMID: 30216510 Retracted.
-
From pancreas to lungs: The role of immune cells in severe acute pancreatitis and acute lung injury.Immun Inflamm Dis. 2024 Jul;12(7):e1351. doi: 10.1002/iid3.1351. Immun Inflamm Dis. 2024. PMID: 39023414 Free PMC article. Review.
-
Signal Pathways and Markers Involved in Acute Lung Injury Induced by Acute Pancreatitis.Dis Markers. 2021 Aug 28;2021:9947047. doi: 10.1155/2021/9947047. eCollection 2021. Dis Markers. 2021. PMID: 34497676 Free PMC article. Review.
Cited by
-
Efficacy and influencing factors of thrombolytic therapy in patients with pulmonary embolism complicated y pulmonary arterial hypertension.Am J Transl Res. 2025 Apr 15;17(4):2654-2664. doi: 10.62347/RJDR4749. eCollection 2025. Am J Transl Res. 2025. PMID: 40385056 Free PMC article.
-
Overexpression of Plakophilin2 Mitigates Capillary Leak Syndrome in Severe Acute Pancreatitis by Activating the p38/MAPK Signaling Pathway.J Inflamm Res. 2024 Jun 26;17:4129-4149. doi: 10.2147/JIR.S459449. eCollection 2024. J Inflamm Res. 2024. PMID: 38952564 Free PMC article.
-
FTO attenuates TNF-α-induced damage of proximal tubular epithelial cells in acute pancreatitis-induced acute kidney injury via targeting AQP3 in an N6-methyladenosine-dependent manner.Ren Fail. 2024 Dec;46(1):2322037. doi: 10.1080/0886022X.2024.2322037. Epub 2024 Mar 6. Ren Fail. 2024. PMID: 38445367 Free PMC article.
-
Intestinal Mucosal Immune Barrier: A Powerful Firewall Against Severe Acute Pancreatitis-Associated Acute Lung Injury via the Gut-Lung Axis.J Inflamm Res. 2024 Apr 10;17:2173-2193. doi: 10.2147/JIR.S448819. eCollection 2024. J Inflamm Res. 2024. PMID: 38617383 Free PMC article. Review.
-
Activin A signaling stimulates neutrophil activation and macrophage migration in pancreatitis.Sci Rep. 2024 Apr 23;14(1):9382. doi: 10.1038/s41598-024-60065-y. Sci Rep. 2024. PMID: 38654064 Free PMC article.
References
-
- Forsmark CE, Vege SS, Wilcox CM. Acute Pancreatitis. N Engl J Med. 2016;375:1972–1981. - PubMed
-
- Banks PA, Bollen TL, Dervenis C, Gooszen HG, Johnson CD, Sarr MG, Tsiotos GG, Vege SS Acute Pancreatitis Classification Working Group. Classification of acute pancreatitis--2012: revision of the Atlanta classification and definitions by international consensus. Gut. 2013;62:102–111. - PubMed
-
- Elder AS, Saccone GT, Dixon DL. Lung injury in acute pancreatitis: mechanisms underlying augmented secondary injury. Pancreatology. 2012;12:49–56. - PubMed
-
- De Campos T, Deree J, Coimbra R. From acute pancreatitis to end-organ injury: mechanisms of acute lung injury. Surg Infect (Larchmt) 2007;8:107–120. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

