Tetramethylpyrazine Alleviates Behavioral and Psychological Symptoms of Dementia Through Facilitating Hippocampal Synaptic Plasticity in Rats With Chronic Cerebral Hypoperfusion
- PMID: 34025340
- PMCID: PMC8134703
- DOI: 10.3389/fnins.2021.646537
Tetramethylpyrazine Alleviates Behavioral and Psychological Symptoms of Dementia Through Facilitating Hippocampal Synaptic Plasticity in Rats With Chronic Cerebral Hypoperfusion
Abstract
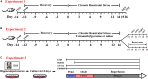
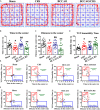
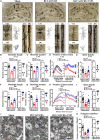
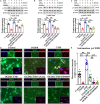
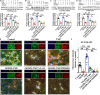
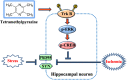
Behavioral and psychological symptoms of dementia (BPSD) ubiquitously disturb all patients with dementia at some point in the disease course. Although a plethora of non-pharmacological and pharmacological methods targeting the relief BPSD have been developed, the therapeutic effect is still far from ideal. Here, a rat BPSD model combining the physiological changes with mental insults was successfully established. Meanwhile, our results indicated that TMP attenuated anxious behavior using an elevated plus maze (EPM) test, ameliorated recognitive ability and sociability through a novel object recognition test (NORT) and social interaction test (SIT), and improved learning and memory impairments via a Barnes maze in rats with bilateral common carotid arteries occlusion (BCCAO) plus chronic restraint stress (CRS). Given that hippocampus chronic cerebral hypoperfusion (CCH) always causes damage to the hippocampus, and the majority of cognitive impairments, behaviors, and stress responses are associated with pathology in the hippocampus including anxiety and depression, we paid attention to investigate the role of the hippocampus in BPSD. Our results indicated that Tetramethylpyrazine (TMP) attenuated anxiety and ameliorated recognitive ability, sociability, learning, and memory impairments due to alleviating dendritic and spine deficits, and upregulating the expression of synapse-related proteins (including PSD95, SYN, GAP43, SYP) in the hippocampus. We also found that the underlying mechanism was that TMP could activate the TrkB/ERK/CREB signaling pathway to promote synaptic remodeling in vivo and in vitro. Mechanically, the present study enlarges the therapeutic scope of TMP in neurodegenerative disorders and provides basic knowledge and feasible candidates for treating BPSD, particularly for vascular dementia.
Keywords: TrkB/ERK/CREB signaling pathway; behavioral and psychological symptoms of dementia; chronic restraint stress; neurodegenerative disorders; synaptic remodeling; tetramethylpyrazine.
Copyright © 2021 Tan, Qiu, Zhang, Yang, Yin, Li, Liu, Li and Yang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





Similar articles
-
Chronic cerebral hypoperfusion induces long-lasting cognitive deficits accompanied by long-term hippocampal silent synapses increase in rats.Behav Brain Res. 2016 Mar 15;301:243-52. doi: 10.1016/j.bbr.2015.12.047. Epub 2016 Jan 3. Behav Brain Res. 2016. PMID: 26756439
-
Neuronal damage, central cholinergic dysfunction and oxidative damage correlate with cognitive deficits in rats with chronic cerebral hypoperfusion.Neurobiol Learn Mem. 2014 Mar;109:7-19. doi: 10.1016/j.nlm.2013.11.016. Epub 2013 Dec 6. Neurobiol Learn Mem. 2014. Retraction in: Neurobiol Learn Mem. 2015 Dec;126:87. doi: 10.1016/j.nlm.2015.11.001. PMID: 24315928 Retracted.
-
Neuroprotective and axonal outgrowth-promoting effects of tetramethylpyrazine nitrone in chronic cerebral hypoperfusion rats and primary hippocampal neurons exposed to hypoxia.Neuropharmacology. 2017 May 15;118:137-147. doi: 10.1016/j.neuropharm.2017.03.022. Epub 2017 Mar 22. Neuropharmacology. 2017. PMID: 28342896
-
Edaravone attenuates oxidative stress induced by chronic cerebral hypoperfusion injury: role of ERK/Nrf2/HO-1 signaling pathway.Neurol Res. 2018 Jan;40(1):1-10. doi: 10.1080/01616412.2017.1376457. Epub 2017 Nov 10. Neurol Res. 2018. PMID: 29125058
-
[Effect of electroacupuncture on expression of CREB and its ability to bind to synaptic proteins in amygdala and hippocampus of rats with post-traumatic stress disorder].Zhen Ci Yan Jiu. 2020 Jul 25;45(7):517-23. doi: 10.13702/j.1000-0607.190709. Zhen Ci Yan Jiu. 2020. PMID: 32705823 Chinese.
Cited by
-
Progress on the Synthesis Pathways and Pharmacological Effects of Naturally Occurring Pyrazines.Molecules. 2024 Jul 30;29(15):3597. doi: 10.3390/molecules29153597. Molecules. 2024. PMID: 39125002 Free PMC article. Review.
-
Neurobiological Mechanisms Underlying Psychological Dysfunction After Brain Injuries.Cells. 2025 Jan 8;14(2):74. doi: 10.3390/cells14020074. Cells. 2025. PMID: 39851502 Free PMC article. Review.
-
The role of Chinese herbal medicine in the regulation of oxidative stress in treating hypertension: from therapeutics to mechanisms.Chin Med. 2024 Oct 29;19(1):150. doi: 10.1186/s13020-024-01022-9. Chin Med. 2024. PMID: 39468572 Free PMC article. Review.
-
Regulatory mechanisms of tetramethylpyrazine on central nervous system diseases: A review.Front Pharmacol. 2022 Sep 5;13:948600. doi: 10.3389/fphar.2022.948600. eCollection 2022. Front Pharmacol. 2022. PMID: 36133805 Free PMC article. Review.
-
Young plasma reverses anesthesia and surgery-induced cognitive impairment in aged rats by modulating hippocampal synaptic plasticity.Front Aging Neurosci. 2022 Sep 6;14:996223. doi: 10.3389/fnagi.2022.996223. eCollection 2022. Front Aging Neurosci. 2022. Retraction in: Front Aging Neurosci. 2023 Dec 15;15:1349869. doi: 10.3389/fnagi.2023.1349869. PMID: 36147703 Free PMC article. Retracted.
References
-
- American Geriatrics Society and American Association for Geriatric Psychiatry (2003). Consensus statement on improving the quality of mental health care in U.S. nursing homes: management of depression and behavioral symptoms associated with dementia. J. Am. Geriatr. Soc. 51 1287–1298. 10.1046/j.1532-5415.2003.51415.x - DOI - PubMed
-
- Ballard C., Corbett A. (2010). Management of neuropsychiatric symptoms in people with dementia. CNS Drugs 24 729–739. - PubMed
-
- Bessey L. J., Walaszek A. (2019). Management of behavioral and psychological symptoms of dementia. Curr. Psychiatry Rep. 21:66. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

