Rod and Cone Connections With Bipolar Cells in the Rabbit Retina
- PMID: 34025360
- PMCID: PMC8134685
- DOI: 10.3389/fncel.2021.662329
Rod and Cone Connections With Bipolar Cells in the Rabbit Retina
Abstract
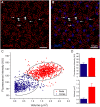
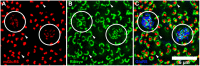
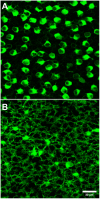
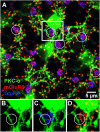
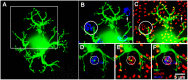
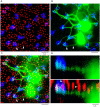
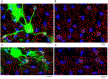
Rod and cone pathways are segregated in the first stage of the retina: cones synapse with both ON- and OFF-cone bipolar cells while rods contact only rod bipolar cells. However, there is an exception to this specific wiring in that rods also contact certain OFF cone bipolar cells, providing a tertiary rod pathway. Recently, it has been proposed that there is even more crossover between rod and cone pathways. Physiological recordings suggested that rod bipolar cells receive input from cones, and ON cone bipolar cells can receive input from rods, in addition to the established pathways. To image their rod and cone contacts, we have dye-filled individual rod bipolar cells in the rabbit retina. We report that approximately half the rod bipolar cells receive one or two cone contacts. Dye-filling AII amacrine cells, combined with subtractive labeling, revealed most of the ON cone bipolar cells to which they were coupled, including the occasional blue cone bipolar cell, identified by its contacts with blue cones. Imaging the AII-coupled ON cone bipolar dendrites in this way showed that they contact cones exclusively. We conclude that there is some limited cone input to rod bipolar cells, but we could find no evidence for rod contacts with ON cone bipolar cells. The tertiary rod OFF pathway operates via direct contacts between rods and OFF cone bipolar cells. In contrast, our results do not support the presence of a tertiary rod ON pathway in the rabbit retina.
Keywords: AII amacrine cell; bipolar cell; cone; retina; rod.
Copyright © 2021 Whitaker, Nobles, Ishibashi and Massey.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
Direct synaptic connections between rods and OFF cone bipolar cells in the rabbit retina.J Comp Neurol. 2004 Jun 14;474(1):1-12. doi: 10.1002/cne.20075. J Comp Neurol. 2004. PMID: 15156575
-
Synaptic connections of the narrow-field, bistratified rod amacrine cell (AII) in the rabbit retina.J Comp Neurol. 1992 Nov 8;325(2):152-68. doi: 10.1002/cne.903250203. J Comp Neurol. 1992. PMID: 1460111
-
Some OFF bipolar cell types make contact with both rods and cones in macaque and mouse retinas.Front Neuroanat. 2014 Sep 26;8:105. doi: 10.3389/fnana.2014.00105. eCollection 2014. Front Neuroanat. 2014. PMID: 25309346 Free PMC article.
-
The neuronal organization of the outer plexiform layer of the primate retina.Int Rev Cytol. 1984;86:285-320. doi: 10.1016/s0074-7696(08)60181-3. Int Rev Cytol. 1984. PMID: 6368448 Review.
-
Distinct synaptic mechanisms create parallel S-ON and S-OFF color opponent pathways in the primate retina.Vis Neurosci. 2014 Mar;31(2):139-51. doi: 10.1017/S0952523813000230. Epub 2013 Jul 29. Vis Neurosci. 2014. PMID: 23895762 Free PMC article. Review.
Cited by
-
Ancient origin of the rod bipolar cell pathway in the vertebrate retina.Res Sq [Preprint]. 2023 Oct 10:rs.3.rs-3411693. doi: 10.21203/rs.3.rs-3411693/v1. Res Sq. 2023. Update in: Nat Ecol Evol. 2024 Jun;8(6):1165-1179. doi: 10.1038/s41559-024-02404-w. PMID: 37886445 Free PMC article. Updated. Preprint.
-
Ancient origin of the rod bipolar cell pathway in the vertebrate retina.Nat Ecol Evol. 2024 Jun;8(6):1165-1179. doi: 10.1038/s41559-024-02404-w. Epub 2024 Apr 16. Nat Ecol Evol. 2024. PMID: 38627529
-
Genetic elimination of rod/cone coupling reveals the contribution of the secondary rod pathway to the retinal output.Sci Adv. 2022 Apr;8(13):eabm4491. doi: 10.1126/sciadv.abm4491. Epub 2022 Apr 1. Sci Adv. 2022. PMID: 35363529 Free PMC article.
-
Rod-cone signal interference in the retina shapes perception in primates.Front Ophthalmol (Lausanne). 2023 Jul 31;3:1230084. doi: 10.3389/fopht.2023.1230084. eCollection 2023. Front Ophthalmol (Lausanne). 2023. PMID: 38983027 Free PMC article.
-
Using optogenetics to dissect rod inputs to OFF ganglion cells in the mouse retina.Front Ophthalmol (Lausanne). 2023;3:1146785. doi: 10.3389/fopht.2023.1146785. Epub 2023 Mar 6. Front Ophthalmol (Lausanne). 2023. PMID: 37426783 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

