Machine Learning for the Diagnosis of Parkinson's Disease: A Review of Literature
- PMID: 34025389
- PMCID: PMC8134676
- DOI: 10.3389/fnagi.2021.633752
Machine Learning for the Diagnosis of Parkinson's Disease: A Review of Literature
Abstract
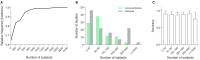
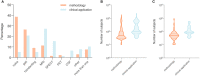
Diagnosis of Parkinson's disease (PD) is commonly based on medical observations and assessment of clinical signs, including the characterization of a variety of motor symptoms. However, traditional diagnostic approaches may suffer from subjectivity as they rely on the evaluation of movements that are sometimes subtle to human eyes and therefore difficult to classify, leading to possible misclassification. In the meantime, early non-motor symptoms of PD may be mild and can be caused by many other conditions. Therefore, these symptoms are often overlooked, making diagnosis of PD at an early stage challenging. To address these difficulties and to refine the diagnosis and assessment procedures of PD, machine learning methods have been implemented for the classification of PD and healthy controls or patients with similar clinical presentations (e.g., movement disorders or other Parkinsonian syndromes). To provide a comprehensive overview of data modalities and machine learning methods that have been used in the diagnosis and differential diagnosis of PD, in this study, we conducted a literature review of studies published until February 14, 2020, using the PubMed and IEEE Xplore databases. A total of 209 studies were included, extracted for relevant information and presented in this review, with an investigation of their aims, sources of data, types of data, machine learning methods and associated outcomes. These studies demonstrate a high potential for adaptation of machine learning methods and novel biomarkers in clinical decision making, leading to increasingly systematic, informed diagnosis of PD.
Keywords: Parkinson's disease; deep learning; diagnosis; differential diagnosis; machine learning.
Copyright © 2021 Mei, Desrosiers and Frasnelli.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

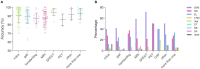
References
-
- Abujrida H., Agu E., Pahlavan K. (2017). Smartphone-based gait assessment to infer Parkinson's disease severity using crowdsourced data, in 2017 IEEE Healthcare Innovations and Point of Care Technologies (HI-POCT) (Bethesda, MD: ), 208–211. 10.1109/HIC.2017.8227621 - DOI
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

