Probiotic Supplementation Facilitates Recovery of 6-OHDA-Induced Motor Deficit via Improving Mitochondrial Function and Energy Metabolism
- PMID: 34025392
- PMCID: PMC8137830
- DOI: 10.3389/fnagi.2021.668775
Probiotic Supplementation Facilitates Recovery of 6-OHDA-Induced Motor Deficit via Improving Mitochondrial Function and Energy Metabolism
Abstract
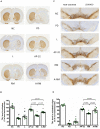
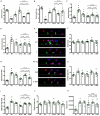
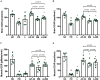
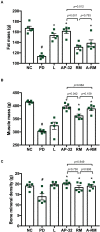
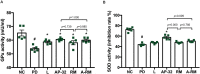
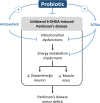
Parkinson's disease (PD) is a neurodegenerative disease associated with progressive impairment of motor and non-motor functions in aging people. Overwhelming evidence indicate that mitochondrial dysfunction is a central factor in PD pathophysiology, which impairs energy metabolism. While, several other studies have shown probiotic supplementations to improve host energy metabolism, alleviate the disease progression, prevent gut microbiota dysbiosis and alter commensal bacterial metabolites. But, whether probiotic and/or prebiotic supplementation can affect energy metabolism and cause the impediment of PD progression remains poorly characterized. Therefore, we investigated 8-weeks supplementation effects of probiotic [Lactobacillus salivarius subsp. salicinius AP-32 (AP-32)], residual medium (RM) obtained from the AP-32 culture medium, and combination of AP-32 and RM (A-RM) on unilateral 6-hydroxydopamine (6-OHDA)-induced PD rats. We found that AP-32, RM and A-RM supplementation induced neuroprotective effects on dopaminergic neurons along with improved motor functions in PD rats. These effects were accompanied by significant increases in mitochondrial activities in the brain and muscle, antioxidative enzymes level in serum, and altered SCFAs profile in fecal samples. Importantly, the AP-32 supplement restored muscle mass along with improved motor function in PD rats, and produced the best results among the supplements. Our results demonstrate that probiotic AP-32 and A-RM supplementations can recover energy metabolism via increasing SCFAs producing and mitochondria function. This restoring of mitochondrial function in the brain and muscles with improved energy metabolism might additionally be potentiated by ROS suppression by the elevated generation of antioxidants, and which finally leads to facilitated recovery of 6-OHDA-induced motor deficit. Taken together, this work demonstrates that probiotic AP-32 supplementation could be a potential candidate for alternate treatment strategy to avert PD progression.
Keywords: 6-hydroxydopamine; Lactobacillus salivarius AP-32; Parkinson’s disease; energy metabolism; mitochondrial function; prebiotic; probiotic.
Copyright © 2021 Nurrahma, Tsao, Wu, Yeh, Hsieh, Panunggal and Huang.
Conflict of interest statement
P-SH was employed by the company Bioflag Biotech Co., Ltd., Tainan City, Taiwan. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Probiotic Enhancement of Antioxidant Capacity and Alterations of Gut Microbiota Composition in 6-Hydroxydopamin-Induced Parkinson's Disease Rats.Antioxidants (Basel). 2021 Nov 17;10(11):1823. doi: 10.3390/antiox10111823. Antioxidants (Basel). 2021. PMID: 34829694 Free PMC article.
-
Long-term Probiotics Intervention Facilitates Recovery of Motor and Non-motor Functions by Regulating Inflammation and Modulating Gut-brain Axis in 6-OHDA Rat Model of Parkinson's Disease.Ann Neurosci. 2025 May 13:09727531251335746. doi: 10.1177/09727531251335746. Online ahead of print. Ann Neurosci. 2025. PMID: 40376431 Free PMC article.
-
Mangosteen Pericarp Extract Supplementation Boosts Antioxidant Status via Rebuilding Gut Microbiota to Attenuate Motor Deficit in 6-OHDA-Induced Parkinson's Disease.Antioxidants (Basel). 2022 Dec 2;11(12):2396. doi: 10.3390/antiox11122396. Antioxidants (Basel). 2022. PMID: 36552604 Free PMC article.
-
Therapeutic potentials of plant iridoids in Alzheimer's and Parkinson's diseases: A review.Eur J Med Chem. 2019 May 1;169:185-199. doi: 10.1016/j.ejmech.2019.03.009. Epub 2019 Mar 8. Eur J Med Chem. 2019. PMID: 30877973 Review.
-
Emerging concepts of mitochondrial dysfunction in Parkinson's disease progression: Pathogenic and therapeutic implications.Mitochondrion. 2020 Jan;50:25-34. doi: 10.1016/j.mito.2019.09.010. Epub 2019 Oct 22. Mitochondrion. 2020. PMID: 31654753 Review.
Cited by
-
PRKN/parkin-mediated mitophagy is induced by the probiotics Saccharomyces boulardii and Lactococcus lactis.Autophagy. 2023 Jul;19(7):2094-2110. doi: 10.1080/15548627.2023.2172873. Epub 2023 Feb 5. Autophagy. 2023. PMID: 36708254 Free PMC article.
-
New hope for Parkinson's disease treatment: Targeting gut microbiota.CNS Neurosci Ther. 2022 Nov;28(11):1675-1688. doi: 10.1111/cns.13916. Epub 2022 Jul 13. CNS Neurosci Ther. 2022. PMID: 35822696 Free PMC article. Review.
-
Lactobacillus rhamnosus GG Modulates Mitochondrial Function and Antioxidant Responses in an Ethanol-Exposed In Vivo Model: Evidence of HIGD2A-Dependent OXPHOS Remodeling in the Liver.Antioxidants (Basel). 2025 May 23;14(6):627. doi: 10.3390/antiox14060627. Antioxidants (Basel). 2025. PMID: 40563263 Free PMC article.
-
Oral Administration of Lactobacillus Inhibits the Permeability of Blood-Brain and Gut Barriers in a Parkinsonism Model.Behav Neurol. 2023 Nov 9;2023:6686037. doi: 10.1155/2023/6686037. eCollection 2023. Behav Neurol. 2023. PMID: 38025189 Free PMC article.
-
Convergent pathways of the gut microbiota-brain axis and neurodegenerative disorders.Gastroenterol Rep (Oxf). 2022 May 16;10:goac017. doi: 10.1093/gastro/goac017. eCollection 2022. Gastroenterol Rep (Oxf). 2022. PMID: 35582476 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources

