Anxious Profile Influences Behavioral and Immunohistological Findings in the Pilocarpine Model of Epilepsy
- PMID: 34025410
- PMCID: PMC8132119
- DOI: 10.3389/fphar.2021.640715
Anxious Profile Influences Behavioral and Immunohistological Findings in the Pilocarpine Model of Epilepsy
Abstract
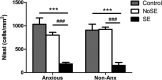
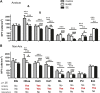
Anxiety and epilepsy have a complex bidirectional relationship, where a depressive/anxious condition is a factor that can trigger seizures which in turn can aggravate the depressive/anxious condition. In addition, brain structures such as the hippocampus and amygdala might have a critical relevance in both epilepsy and anxiety. The aim of the present work was to investigate the influence of different anxious profiles to epileptogenesis. Initially, animals were screened through the elevated plus-maze anxiety test, and then seizure development was evaluated using the pilocarpine model of epilepsy. There were no differences in the susceptibility to status epilepticus, mortality rate or frequency of spontaneous recurrent seizures between animals characterized as anxious as compared to the non-anxious animals. Next, we evaluated immunohistological patterns related to seizures and anxiety in various related brain areas. Despite a decrease in the density of neuropeptide Y and parvalbumin expression in epileptic animals, those presenting greater neuropeptide Y immunoreactivity in various brain regions, also showed higher spontaneous recurrent seizures frequency. Differences on the anxious profile showed to interfere with some of these findings in some regions. In addition, animals that were injected with pilocarpine, but did not develop status epilepticus, had behavioral and neuroanatomical alterations as compared to control animals, indicating its importance as an additional tool for investigating the heterogeneity of the epileptogenic response after an initial insult. This study allowed to better understand the association between anxiety and temporal lobe epilepsy and might allow for therapeutic targets to be developed to minimize the negative impacts associated with it.
Keywords: epileptogenesis; neuropeptide y; parvalbumin; seizure; temporal lobe epilepsy.
Copyright © 2021 Kohek, Foresti, Blanco, Cavarsan, Silva and Mello.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
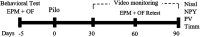

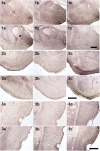
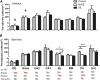
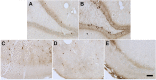
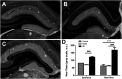
Similar articles
-
The frequency of spontaneous seizures in rats correlates with alterations in sensorimotor gating, spatial working memory, and parvalbumin expression throughout limbic regions.Neuroscience. 2016 Jan 15;312:86-98. doi: 10.1016/j.neuroscience.2015.11.008. Epub 2015 Nov 12. Neuroscience. 2016. PMID: 26582750
-
Behavioral and cognitive alterations, spontaneous seizures, and neuropathology developing after a pilocarpine-induced status epilepticus in C57BL/6 mice.Exp Neurol. 2009 Sep;219(1):284-97. doi: 10.1016/j.expneurol.2009.05.035. Epub 2009 Jun 3. Exp Neurol. 2009. PMID: 19500573
-
Behavioral alterations in the pilocarpine model of temporal lobe epilepsy in mice.Exp Neurol. 2007 Oct;207(2):329-49. doi: 10.1016/j.expneurol.2007.06.021. Epub 2007 Jul 12. Exp Neurol. 2007. PMID: 17714705
-
Animal models of epilepsy for the development of antiepileptogenic and disease-modifying drugs. A comparison of the pharmacology of kindling and post-status epilepticus models of temporal lobe epilepsy.Epilepsy Res. 2002 Jun;50(1-2):105-23. doi: 10.1016/s0920-1211(02)00073-6. Epilepsy Res. 2002. PMID: 12151122 Review.
-
Animal models of epilepsy: use and limitations.Neuropsychiatr Dis Treat. 2014 Sep 9;10:1693-705. doi: 10.2147/NDT.S50371. eCollection 2014. Neuropsychiatr Dis Treat. 2014. PMID: 25228809 Free PMC article. Review.
Cited by
-
The inhibition of PGAM5 suppresses seizures in a kainate-induced epilepsy model via mitophagy reduction.Front Mol Neurosci. 2022 Dec 22;15:1047801. doi: 10.3389/fnmol.2022.1047801. eCollection 2022. Front Mol Neurosci. 2022. PMID: 36618822 Free PMC article.
-
Impact of LITAF on Mitophagy and Neuronal Damage in Epilepsy via MCL-1 Ubiquitination.CNS Neurosci Ther. 2025 Jan;31(1):e70191. doi: 10.1111/cns.70191. CNS Neurosci Ther. 2025. PMID: 39764629 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources

