Curcumin as a Potential Treatment for COVID-19
- PMID: 34025433
- PMCID: PMC8138567
- DOI: 10.3389/fphar.2021.675287
Curcumin as a Potential Treatment for COVID-19
Abstract
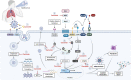
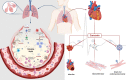
Coronavirus disease 2019 (COVID-19) is an infectious disease that rapidly spread throughout the world leading to high mortality rates. Despite the knowledge of previous diseases caused by viruses of the same family, such as MERS and SARS-CoV, management and treatment of patients with COVID-19 is a challenge. One of the best strategies around the world to help combat the COVID-19 has been directed to drug repositioning; however, these drugs are not specific to this new virus. Additionally, the pathophysiology of COVID-19 is highly heterogeneous, and the way of SARS-CoV-2 modulates the different systems in the host remains unidentified, despite recent discoveries. This complex and multifactorial response requires a comprehensive therapeutic approach, enabling the integration and refinement of therapeutic responses of a given single compound that has several action potentials. In this context, natural compounds, such as Curcumin, have shown beneficial effects on the progression of inflammatory diseases due to its numerous action mechanisms: antiviral, anti-inflammatory, anticoagulant, antiplatelet, and cytoprotective. These and many other effects of curcumin make it a promising target in the adjuvant treatment of COVID-19. Hence, the purpose of this review is to specifically point out how curcumin could interfere at different times/points during the infection caused by SARS-CoV-2, providing a substantial contribution of curcumin as a new adjuvant therapy for the treatment of COVID-19.
Keywords: ACE2; COVID-19; SARS-CoV-2; curcumin; new therapies.
Copyright © 2021 Rattis, Ramos and Celes.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Almatroodi S. A., Alrumaihi F., Alsahli M. A., Alhommrani M. F., Khan A., Rahmani A. H. (2020). Curcumin, an Active Constituent of Turmeric Spice: Implication in the Prevention of Lung Injury Induced by Benzo(a) Pyrene (BAP) in Rats. Molecules 25, 724–819. 10.3390/molecules25030724 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

