Delivery of Rice Gall Dwarf Virus Into Plant Phloem by Its Leafhopper Vectors Activates Callose Deposition to Enhance Viral Transmission
- PMID: 34025616
- PMCID: PMC8132966
- DOI: 10.3389/fmicb.2021.662577
Delivery of Rice Gall Dwarf Virus Into Plant Phloem by Its Leafhopper Vectors Activates Callose Deposition to Enhance Viral Transmission
Abstract
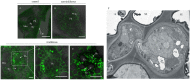
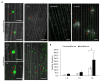
Rice gall dwarf virus (RGDV) and its leafhopper vector Recilia dorsalis are plant phloem-inhabiting pests. Currently, how the delivery of plant viruses into plant phloem via piercing-sucking insects modulates callose deposition to promote viral transmission remains poorly understood. Here, we initially demonstrated that nonviruliferous R. dorsalis preferred feeding on RGDV-infected rice plants than viruliferous counterpart. Electrical penetration graph assay showed that viruliferous R. dorsalis encountered stronger physical barriers than nonviruliferous insects during feeding, finally prolonging salivary secretion and ingestion probing. Viruliferous R. dorsalis feeding induced more defense-associated callose deposition on sieve plates of rice phloem. Furthermore, RGDV infection significantly increased the cytosolic Ca2+ level in rice plants, triggering substantial callose deposition. Such a virus-mediated insect feeding behavior change potentially impedes insects from continuously ingesting phloem sap and promotes the secretion of more infectious virions from the salivary glands into rice phloem. This is the first study demonstrating that the delivery of a phloem-limited virus by piercing-sucking insects into the plant phloem activates the defense-associated callose deposition to enhance viral transmission.
Keywords: callose deposition; callose synthase genes; insect feeding behavior; phloem-limited virus; rice leafhopper.
Copyright © 2021 Yi, Wu and Wei.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
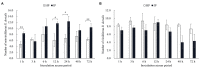




Similar articles
-
Rice Gall Dwarf Virus Promotes the Propagation and Transmission of Rice Stripe Mosaic Virus by Co-infected Insect Vectors.Front Microbiol. 2022 Feb 11;13:834712. doi: 10.3389/fmicb.2022.834712. eCollection 2022. Front Microbiol. 2022. PMID: 35222343 Free PMC article.
-
A leafhopper saliva protein mediates horizontal transmission of viral pathogens from insect vectors into rice phloem.Commun Biol. 2022 Mar 4;5(1):204. doi: 10.1038/s42003-022-03160-y. Commun Biol. 2022. PMID: 35246603 Free PMC article.
-
Adverse Effects of Rice gall dwarf virus upon its Insect Vector Recilia dorsalis (Hemiptera: Cicadellidae).Plant Dis. 2016 Apr;100(4):784-790. doi: 10.1094/PDIS-06-15-0713-RE. Epub 2016 Feb 4. Plant Dis. 2016. PMID: 30688603
-
Sieve element occlusion: Interactions with phloem sap-feeding insects. A review.J Plant Physiol. 2022 Feb;269:153582. doi: 10.1016/j.jplph.2021.153582. Epub 2021 Dec 5. J Plant Physiol. 2022. PMID: 34953413 Review.
-
How phloem-feeding insects face the challenge of phloem-located defenses.Front Plant Sci. 2013 Aug 29;4:336. doi: 10.3389/fpls.2013.00336. Front Plant Sci. 2013. PMID: 24009620 Free PMC article. Review.
Cited by
-
Plant viruses exploit insect salivary GAPDH to modulate plant defenses.Nat Commun. 2024 Aug 12;15(1):6918. doi: 10.1038/s41467-024-51369-8. Nat Commun. 2024. PMID: 39134555 Free PMC article.
-
The multifarious role of callose and callose synthase in plant development and environment interactions.Front Plant Sci. 2023 May 31;14:1183402. doi: 10.3389/fpls.2023.1183402. eCollection 2023. Front Plant Sci. 2023. PMID: 37324665 Free PMC article. Review.
-
Leafhopper salivary vitellogenin mediates virus transmission to plant phloem.Nat Commun. 2024 Jan 2;15(1):3. doi: 10.1038/s41467-023-43488-5. Nat Commun. 2024. PMID: 38167823 Free PMC article.
-
Variation of endosymbiont and citrus tristeza virus (CTV) titers in the Huanglongbing insect vector, Diaphorina citri, on CTV-infected plants.Front Microbiol. 2023 Sep 22;14:1236731. doi: 10.3389/fmicb.2023.1236731. eCollection 2023. Front Microbiol. 2023. PMID: 37808301 Free PMC article.
-
Exploring Virus Diversity in the Potato leafhopper (Empoasca fabae), an Economically Important Agricultural Pest.Viruses. 2024 Aug 16;16(8):1305. doi: 10.3390/v16081305. Viruses. 2024. PMID: 39205279 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Miscellaneous

