Heat Shock Protein A6, a Novel HSP70, Is Induced During Enterovirus A71 Infection to Facilitate Internal Ribosomal Entry Site-Mediated Translation
- PMID: 34025620
- PMCID: PMC8137988
- DOI: 10.3389/fmicb.2021.664955
Heat Shock Protein A6, a Novel HSP70, Is Induced During Enterovirus A71 Infection to Facilitate Internal Ribosomal Entry Site-Mediated Translation
Abstract
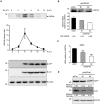
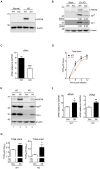
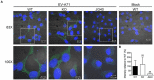
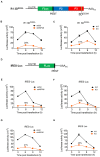
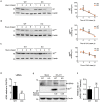
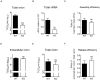
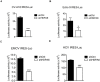
Enterovirus A71 (EV-A71) is a human pathogen causing hand, foot, and mouth disease (HFMD) in children. Its infection can lead to severe neurological diseases or even death in some cases. While being produced in a large quantity during infection, viral proteins often require the assistance from cellular chaperones for proper folding. In this study, we found that heat shock protein A6 (HSPA6), whose function in viral life cycle is scarcely studied, was induced and functioned as a positive regulator for EV-A71 infection. Depletion of HSPA6 led to the reductions of EV-A71 viral proteins, viral RNA and virions as a result of the downregulation of internal ribosomal entry site (IRES)-mediated translation. Unlike other HSP70 isoforms such as HSPA1, HSPA8, and HSPA9, which regulate all phases of the EV-A71 life, HSPA6 was required for the IRES-mediated translation only. Unexpectedly, the importance of HSPA6 in the IRES activity could be observed in the absence of viral proteins, suggesting that HSPA6 facilitated IRES activity through cellular factor(s) instead of viral proteins. Intriguingly, the knockdown of HSPA6 also caused the reduction of luciferase activity driven by the IRES from coxsackievirus A16, echovirus 9, encephalomyocarditis virus, or hepatitis C virus, supporting that HSPA6 may assist the function of a cellular protein generally required for viral IRES activities.
Keywords: HSPA6; enterovirus A71 (EV-A71); induced HSP70; internal ribosomal entry site; viral IRES.
Copyright © 2021 Su, Hwang and Chen.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Basavappa R., Syed R., Flore O., Icenogle J. P., Filman D. J., Hogle J. M. (1994). Role and mechanism of the maturation cleavage of VP0 in poliovirus assembly: structure of the empty capsid assembly intermediate at 2.9 A resolution. Protein Sci. 3 1651–1669. 10.1002/pro.5560031005 - DOI - PMC - PubMed
-
- Bolhassani A., Agi E. (2019). Heat shock proteins in infection. Clin. Chim. Acta 498 90–100. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

