Epidermal Growth Factor Modulates Palmitic Acid-Induced Inflammatory and Lipid Signaling Pathways in SZ95 Sebocytes
- PMID: 34025636
- PMCID: PMC8134683
- DOI: 10.3389/fimmu.2021.600017
Epidermal Growth Factor Modulates Palmitic Acid-Induced Inflammatory and Lipid Signaling Pathways in SZ95 Sebocytes
Abstract
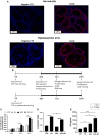
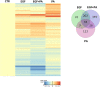
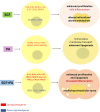
Epidermal growth factor (EGF) acts as a paracrine and autocrine mediator of cell proliferation and differentiation in various types of epithelial cells, such as sebocytes, which produce the lipid-rich sebum to moisturize the skin. However, sebum lipids via direct contact and by penetrating through the epidermis may have regulatory roles on epidermal and dermal cells as well. As EGF receptor (EGFR) is expressed throughout the proliferating and the lipid-producing layers of sebaceous glands (SGs) in healthy and acne-involved skin, we investigated the effect of EGF on SZ95 sebocytes and how it may alter the changes induced by palmitic acid (PA), a major sebum component with bioactive roles. We found that EGF is not only a potent stimulator of sebocyte proliferation, but also induces the secretion of interleukin (IL)6 and down-regulates the expression of genes involved in steroid and retinoid metabolism. Importantly, when applied in combination with PA, the PA-induced lipid accumulation was decreased and the cells secreted increased IL6 levels. Functional clustering of the differentially regulated genes in SZ95 sebocytes treated with EGF, PA or co-treated with EGF+PA further confirmed that EGF may be a potent inducer of hyperproliferative/inflammatory pathways (IL1 signaling), an effect being more pronounced in the presence of PA. However, while a group of inflammatory genes was up-regulated significantly in EGF+PA co-treated sebocytes, PA treatment in the absence of EGF, regulated genes only related to cell homeostasis. Meta-analysis of the gene expression profiles of whole acne tissue samples and EGF- and EGF+PA -treated SZ95 sebocytes showed that the EGF+PA co-activation of sebocytes may also have implications in disease. Altogether, our results reveal that PA-induced lipid accumulation and inflammation can be modulated by EGF in sebocytes, which also highlights the need for system biological approaches to better understand sebaceous (immuno)biology.
Keywords: EGF; acne; palmitic acid; sebocytes; sebum.
Copyright © 2021 Törőcsik, Fazekas, Póliska, Gregus, Janka, Dull, Szegedi, Zouboulis and Kovács.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





Similar articles
-
Linoleic Acid Induced Changes in SZ95 Sebocytes-Comparison with Palmitic Acid and Arachidonic Acid.Nutrients. 2023 Jul 26;15(15):3315. doi: 10.3390/nu15153315. Nutrients. 2023. PMID: 37571253 Free PMC article.
-
Enhancement of lipid content and inflammatory cytokine secretion in SZ95 sebocytes by palmitic acid suggests a potential link between free fatty acids and acne aggravation.Exp Dermatol. 2019 Feb;28(2):207-210. doi: 10.1111/exd.13855. Exp Dermatol. 2019. PMID: 30506807
-
Increased Lipid Accumulation under Hypoxia in SZ95 Human Sebocytes.Dermatology. 2021;237(1):131-141. doi: 10.1159/000505537. Epub 2020 Feb 21. Dermatology. 2021. PMID: 32088721
-
Acne and sebaceous gland function.Clin Dermatol. 2004 Sep-Oct;22(5):360-6. doi: 10.1016/j.clindermatol.2004.03.004. Clin Dermatol. 2004. PMID: 15556719 Review.
-
Lipid droplets and associated proteins in sebocytes.Exp Cell Res. 2016 Jan 15;340(2):205-8. doi: 10.1016/j.yexcr.2015.11.008. Epub 2015 Nov 11. Exp Cell Res. 2016. PMID: 26571075 Review.
Cited by
-
New insights into the role of Cutibacterium acnes-derived extracellular vesicles in inflammatory skin disorders.Sci Rep. 2023 Sep 25;13(1):16058. doi: 10.1038/s41598-023-43354-w. Sci Rep. 2023. PMID: 37749255 Free PMC article.
-
Stable knockdown of Drp1 improves retinoic acid-BDNF-induced neuronal differentiation through global transcriptomic changes and results in reduced phosphorylation of ERK1/2 independently of DUSP1 and 6.Front Cell Dev Biol. 2024 Mar 14;12:1342741. doi: 10.3389/fcell.2024.1342741. eCollection 2024. Front Cell Dev Biol. 2024. PMID: 38550381 Free PMC article.
-
Potential Cosmetic Applications of the Combined Extract of Panax ginseng, Ganoderma lucidum, Cordyceps militaris, and Several Asian Plants.J Cosmet Dermatol. 2025 Aug;24(8):e70343. doi: 10.1111/jocd.70343. J Cosmet Dermatol. 2025. PMID: 40762221 Free PMC article.
-
Linoleic Acid Induced Changes in SZ95 Sebocytes-Comparison with Palmitic Acid and Arachidonic Acid.Nutrients. 2023 Jul 26;15(15):3315. doi: 10.3390/nu15153315. Nutrients. 2023. PMID: 37571253 Free PMC article.
-
Protective Effects of Naringenin and Apigenin in Ameliorating Skin Damage via Mediating the Nrf2 and NF-κB Pathways in Mice.Foods. 2023 May 24;12(11):2120. doi: 10.3390/foods12112120. Foods. 2023. PMID: 37297362 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

