Integrating CAR T-Cell Therapy and Transplantation: Comparisons of Safety and Long-Term Efficacy of Allogeneic Hematopoietic Stem Cell Transplantation After CAR T-Cell or Chemotherapy-Based Complete Remission in B-Cell Acute Lymphoblastic Leukemia
- PMID: 34025637
- PMCID: PMC8138447
- DOI: 10.3389/fimmu.2021.605766
Integrating CAR T-Cell Therapy and Transplantation: Comparisons of Safety and Long-Term Efficacy of Allogeneic Hematopoietic Stem Cell Transplantation After CAR T-Cell or Chemotherapy-Based Complete Remission in B-Cell Acute Lymphoblastic Leukemia
Abstract
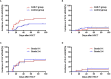
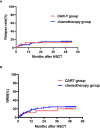
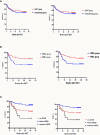
Patients often undergo consolidation allogeneic hematopoietic stem cell transplantation (allo-HSCT) to maintain long-term remission following chimeric antigen receptor (CAR) T-cell therapy. Comparisons of safety and efficacy of allo-HSCT following complete remission (CR) achieved by CAR-T therapy versus by chemotherapy for B-cell acute lymphoblastic leukemia (B-ALL) has not been reported. We performed a parallel comparison of transplant outcomes in 105 consecutive B-ALL patients who received allo-HSCT after achieving CR with CAR-T therapy (n=27) or with chemotherapy (n=78). The CAR-T-allo-HSCT group had more patients in second CR compared to the chemotherapy-allo-HSCT group (78% vs. 37%; p<0.01) and more with complex cytogenetics (44% vs. 6%; p<0.001) but the proportion of patients with pre-transplant minimal residual disease (MRD) was similar. The median follow-up time was 49 months (range: 25-54 months). The CAR-T cohort had a higher incidence of Grade II-IV acute graft-versus-host disease (aGVHD 48.1% [95% CI: 46.1-50.1%] vs. 25.6% [95%CI: 25.2-26.0%]; p=0.016). The incidence of Grade III-IV aGVHD was similar in both groups (11.1% vs.11.5%, p=0.945). The overall incidence of chronic GVHD in the CAR-T group was higher compared to the chemotherapy group (73.3% [95%CI: 71.3-75.3%] vs. 55.0% [95%CI: 54.2-55.8%], p=0.107), but the rate of extensive chronic GVHD was similar (11.1% vs.11.9%, p=0.964). Efficacy measures 4 years following transplant were all similar in the CAR-T vs. the chemotherapy groups: cumulative incidences of relapse (CIR; 11.1% vs.12.8%; p=0.84), cumulative incidences of non-relapse mortality (NRM; 18.7% vs. 23.1%; p=0.641) leukemia-free survival (LFS; 70.2% vs. 64.1%; p=0.63) and overall survival (OS; 70.2% vs. 65.4%; p=0.681). We found that pre-transplant MRD-negative CR predicted a lower CIR and a higher LFS compared with MRD-positive CR. In conclusion, our data indicate that, in B-ALL patients, similar clinical safety outcomes could be achieved with either CD19 CAR T-cell therapy followed by allo-HSCT or chemotherapy followed by allo-HSCT. Despite the inclusion of more patients with advanced diseases in the CAR-T group, the 4-year LFS and OS achieved with CAR T-cells followed by allo-HSCT were as remarkable as those achieved with chemotherapy followed by allo-HSCT. Further confirmation of these results requires larger, randomized clinical trials.
Keywords: CD19 CAR T-cell therapy; allogeneic hematopoietic cell transplantation; relapse; relapse/refractory B cell acute lymphoblastic leukemia; survival.
Copyright © 2021 Zhao, Liu, Sun, Zhang, Zhou, Wei, Xiong, Cao, Lu, Yang, Zhang, Lu and Lu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Comparable outcomes in patients with B-cell acute lymphoblastic leukemia receiving haploidentical hematopoietic stem cell transplantation: Pretransplant minimal residual disease-negative complete remission following chimeric antigen receptor T-cell therapy versus chemotherapy.Front Immunol. 2022 Aug 30;13:934442. doi: 10.3389/fimmu.2022.934442. eCollection 2022. Front Immunol. 2022. PMID: 36110859 Free PMC article.
-
Humanized Anti-CD19 CAR-T Cell Therapy and Sequential Allogeneic Hematopoietic Stem Cell Transplantation Achieved Long-Term Survival in Refractory and Relapsed B Lymphocytic Leukemia: A Retrospective Study of CAR-T Cell Therapy.Front Immunol. 2021 Oct 29;12:755549. doi: 10.3389/fimmu.2021.755549. eCollection 2021. Front Immunol. 2021. PMID: 34777367 Free PMC article.
-
Analysis benefits of a second Allo-HSCT after CAR-T cell therapy in patients with relapsed/refractory B-cell acute lymphoblastic leukemia who relapsed after transplant.Front Immunol. 2023 Jul 4;14:1191382. doi: 10.3389/fimmu.2023.1191382. eCollection 2023. Front Immunol. 2023. PMID: 37469510 Free PMC article.
-
How to Combine the Two Landmark Treatment Methods-Allogeneic Hematopoietic Stem Cell Transplantation and Chimeric Antigen Receptor T Cell Therapy Together to Cure High-Risk B Cell Acute Lymphoblastic Leukemia?Front Immunol. 2020 Dec 15;11:611710. doi: 10.3389/fimmu.2020.611710. eCollection 2020. Front Immunol. 2020. PMID: 33384696 Free PMC article. Review.
-
Efficacy and safety of CD19 CAR-T cell therapy for acute lymphoblastic leukemia patients relapsed after allogeneic hematopoietic stem cell transplantation.Int J Hematol. 2022 Sep;116(3):315-329. doi: 10.1007/s12185-022-03398-6. Epub 2022 Jun 23. Int J Hematol. 2022. PMID: 35737192 Review.
Cited by
-
Comparable outcomes in patients with B-cell acute lymphoblastic leukemia receiving haploidentical hematopoietic stem cell transplantation: Pretransplant minimal residual disease-negative complete remission following chimeric antigen receptor T-cell therapy versus chemotherapy.Front Immunol. 2022 Aug 30;13:934442. doi: 10.3389/fimmu.2022.934442. eCollection 2022. Front Immunol. 2022. PMID: 36110859 Free PMC article.
-
Treatment strategies for relapse after CAR T-cell therapy in B cell lymphoma.Front Pediatr. 2024 Jan 12;11:1305657. doi: 10.3389/fped.2023.1305657. eCollection 2023. Front Pediatr. 2024. PMID: 38283399 Free PMC article. Review.
-
CD19/CD22 dual-targeting chimeric antigen receptor T-cell therapy bridging to allogeneic haematopoietic stem cell transplantation for B-cell acute lymphoblastic leukaemia delays platelet recovery and increases risks of cytomegalovirus and Epstein-Barr virus viremia after transplantation.Clin Transl Med. 2023 Oct;13(10):e1459. doi: 10.1002/ctm2.1459. Clin Transl Med. 2023. PMID: 37882317 Free PMC article. No abstract available.
-
PET/CT Evaluation of the Effect of Allogeneic Hematopoietic Stem Cell Transplantation in the Treatment of T-Cell Lymphoblastic Lymphoma.Contrast Media Mol Imaging. 2022 Aug 16;2022:6057017. doi: 10.1155/2022/6057017. eCollection 2022. Contrast Media Mol Imaging. 2022. PMID: 36072622 Free PMC article. Review.
-
Prognostic factors of second hematopoietic allogeneic stem cell transplantation among hematological malignancy patients relapsed after first hematopoietic stem cell transplantation: A single center study.Front Immunol. 2023 Jan 4;13:1066748. doi: 10.3389/fimmu.2022.1066748. eCollection 2022. Front Immunol. 2023. PMID: 36685540 Free PMC article.
References
-
- Zhao Y, Tong W, Cao X-y, Xiong M, Zhang J, Wei Z, et al. . Improved Outcomes of Haploidentical Blood and Marrow Transplantation in Hematologic Malignancies: A Single Center Study of 514 Cases. Blood (2015) 126:3224. 10.1182/blood.V126.23.3224.3224 - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

