Attenuated T Cell Responses Are Associated With the Blockade of Cerebral Malaria Development by YOP1-Deficient Plasmodium berghei ANKA
- PMID: 34025654
- PMCID: PMC8134684
- DOI: 10.3389/fimmu.2021.642585
Attenuated T Cell Responses Are Associated With the Blockade of Cerebral Malaria Development by YOP1-Deficient Plasmodium berghei ANKA
Abstract
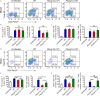
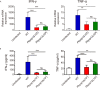
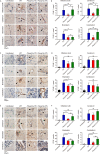
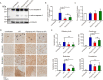
Reticulon and the REEP family of proteins stabilize the high curvature of endoplasmic reticulum tubules. The REEP5 homolog in Plasmodium, Plasmodium berghei YOP1 (PbYOP1), plays an important role in the erythrocytic cycle of the P. berghei ANKA and the pathogenesis of experimental cerebral malaria (ECM), but the mechanisms are largely unknown. Here, we show that protection from ECM in Pbyop1Δ-infected mice is associated with reduced intracerebral Th1 accumulation, decreased expression of pro-inflammatory cytokines and chemokines, and attenuated pathologies in the brainstem, though the total number of CD4+ and CD8+ T cells sequestered in the brain are not reduced. Expression of adhesive molecules on brain endothelial cells, including ICAM-1, VCAM-1, and CD36, are decreased, particularly in the brainstem, where fatal pathology is always induced during ECM. Subsequently, CD8+ T cell-mediated cell apoptosis in the brain is compromised. These findings suggest that Pbyop1Δ parasites can be a useful tool for mechanistic investigation of cerebral malaria pathogenesis.
Keywords: Plasmodium berghei; T cell; YOP1; cerebral malaria; immune response.
Copyright © 2021 Hai, Shi and Wang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




Similar articles
-
A Plasmodium homolog of ER tubule-forming proteins is required for parasite virulence.Mol Microbiol. 2020 Sep;114(3):454-467. doi: 10.1111/mmi.14526. Epub 2020 Jun 19. Mol Microbiol. 2020. PMID: 32432369 Free PMC article.
-
Mycobacterium tuberculosis Coinfection Has No Impact on Plasmodium berghei ANKA-Induced Experimental Cerebral Malaria in C57BL/6 Mice.Infect Immun. 2015 Dec 7;84(2):502-10. doi: 10.1128/IAI.01290-15. Print 2016 Feb. Infect Immun. 2015. PMID: 26644378 Free PMC article.
-
Type I interferons contribute to experimental cerebral malaria development in response to sporozoite or blood-stage Plasmodium berghei ANKA.Eur J Immunol. 2013 Oct;43(10):2683-95. doi: 10.1002/eji.201343327. Epub 2013 Jul 19. Eur J Immunol. 2013. PMID: 23780878
-
Unravelling the roles of innate lymphoid cells in cerebral malaria pathogenesis.Parasite Immunol. 2018 Feb;40(2). doi: 10.1111/pim.12502. Parasite Immunol. 2018. PMID: 29117626 Review.
-
Genetic analysis of cerebral malaria in the mouse model infected with Plasmodium berghei.Mamm Genome. 2018 Aug;29(7-8):488-506. doi: 10.1007/s00335-018-9752-9. Epub 2018 Jun 19. Mamm Genome. 2018. PMID: 29922917 Review.
Cited by
-
Mannose inhibits Plasmodium parasite growth and cerebral malaria development via regulation of host immune responses.Front Immunol. 2022 Sep 23;13:859228. doi: 10.3389/fimmu.2022.859228. eCollection 2022. Front Immunol. 2022. PMID: 36211381 Free PMC article.
References
-
- World Health Organization . World Malaria Report 2020. Geneva: World Health Organization; (2020).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

