In Situ Vaccination as a Strategy to Modulate the Immune Microenvironment of Hepatocellular Carcinoma
- PMID: 34025657
- PMCID: PMC8137829
- DOI: 10.3389/fimmu.2021.650486
In Situ Vaccination as a Strategy to Modulate the Immune Microenvironment of Hepatocellular Carcinoma
Abstract
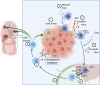
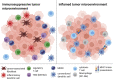
Hepatocellular Carcinoma (HCC) is a highly prevalent malignancy that develops in patients with chronic liver diseases and dysregulated systemic and hepatic immunity. The tumor microenvironment (TME) contains tumor-associated macrophages (TAM), cancer-associated fibroblasts (CAF), regulatory T cells (Treg) and myeloid-derived suppressor cells (MDSC) and is central to mediating immune evasion and resistance to therapy. The interplay between these cells types often leads to insufficient antigen presentation, preventing effective anti-tumor immune responses. In situ vaccines harness the tumor as the source of antigens and implement sequential immunomodulation to generate systemic and lasting antitumor immunity. Thus, in situ vaccines hold the promise to induce a switch from an immunosuppressive environment where HCC cells evade antigen presentation and suppress T cell responses towards an immunostimulatory environment enriched for activated cytotoxic cells. Pivotal steps of in situ vaccination include the induction of immunogenic cell death of tumor cells, a recruitment of antigen-presenting cells with a focus on dendritic cells, their loading and maturation and a subsequent cross-priming of CD8+ T cells to ensure cytotoxic activity against tumor cells. Several in situ vaccine approaches have been suggested, with vaccine regimens including oncolytic viruses, Flt3L, GM-CSF and TLR agonists. Moreover, combinations with checkpoint inhibitors have been suggested in HCC and other tumor entities. This review will give an overview of various in situ vaccine strategies for HCC, highlighting the potentials and pitfalls of in situ vaccines to treat liver cancer.
Keywords: dendritic cells (DC); hepatocellular carcinoma (HCC); immunotherapy; in situ vaccine; tumor microenvironment.
Copyright © 2021 Lurje, Werner, Mohr, Roderburg, Tacke and Hammerich.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Emergence of immunotherapy as a novel way to treat hepatocellular carcinoma.World J Gastroenterol. 2018 May 7;24(17):1839-1858. doi: 10.3748/wjg.v24.i17.1839. World J Gastroenterol. 2018. PMID: 29740200 Free PMC article. Review.
-
Immune microenvironment and immunotherapy in hepatocellular carcinoma: mechanisms and advances.Front Immunol. 2025 Apr 2;16:1581098. doi: 10.3389/fimmu.2025.1581098. eCollection 2025. Front Immunol. 2025. PMID: 40242773 Free PMC article. Review.
-
Cancer-associated fibroblast-targeted strategy enhances antitumor immune responses in dendritic cell-based vaccine.Cancer Sci. 2015 Feb;106(2):134-42. doi: 10.1111/cas.12584. Epub 2015 Jan 16. Cancer Sci. 2015. PMID: 25483888 Free PMC article.
-
ATR inhibitor AZD6738 enhances the antitumor activity of radiotherapy and immune checkpoint inhibitors by potentiating the tumor immune microenvironment in hepatocellular carcinoma.J Immunother Cancer. 2020 May;8(1):e000340. doi: 10.1136/jitc-2019-000340. J Immunother Cancer. 2020. PMID: 32461345 Free PMC article.
-
An autologous in situ tumor vaccination approach for hepatocellular carcinoma. 2. Tumor-specific immunity and cure after radio-inducible suicide gene therapy and systemic CD40-ligand and Flt3-ligand gene therapy in an orthotopic tumor model.Radiat Res. 2014 Aug;182(2):201-10. doi: 10.1667/RR13617.1. Epub 2014 Jul 3. Radiat Res. 2014. PMID: 24992166
Cited by
-
Theoretical premises of a "three in one" therapeutic approach to treat immunogenic and nonimmunogenic cancers: a narrative review.Transl Cancer Res. 2021 Nov;10(11):4958-4972. doi: 10.21037/tcr-21-919. Transl Cancer Res. 2021. PMID: 35116346 Free PMC article. Review.
-
Construction of a Ferroptosis-Related Nine-lncRNA Signature for Predicting Prognosis and Immune Response in Hepatocellular Carcinoma.Front Immunol. 2021 Sep 17;12:719175. doi: 10.3389/fimmu.2021.719175. eCollection 2021. Front Immunol. 2021. PMID: 34603293 Free PMC article.
-
Interventional Oncology Meets Immuno-oncology: Combination Therapies for Hepatocellular Carcinoma.Radiology. 2024 Nov;313(2):e232875. doi: 10.1148/radiol.232875. Radiology. 2024. PMID: 39560477 Review.
-
Comprehensive analysis of cuproptosis-related lncRNAs for prognostic significance and immune microenvironment characterization in hepatocellular carcinoma.Front Immunol. 2023 Jan 4;13:991604. doi: 10.3389/fimmu.2022.991604. eCollection 2022. Front Immunol. 2023. PMID: 36685508 Free PMC article.
-
Prodrugs of paclitaxel improve in situ photo-vaccination.Photochem Photobiol. 2024 Oct 9:10.1111/php.14025. doi: 10.1111/php.14025. Online ahead of print. Photochem Photobiol. 2024. PMID: 39384406
References
-
- Murray CJ, Vos T, Lozano R, Naghavi M, Flaxman AD, Michaud C, et al. . Disability-Adjusted Life Years (DALYs) for 291 Diseases and Injuries in 21 Regions, 1990-2010: A Systematic Analysis for the Global Burden of Disease Study 2010. Lancet (London England) (2012) 380(9859):2197–223. 10.1016/s0140-6736(12)61689-4 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

