Epigenetic Regulation of BST-2 Expression Levels and the Effect on HIV-1 Pathogenesis
- PMID: 34025670
- PMCID: PMC8131512
- DOI: 10.3389/fimmu.2021.669241
Epigenetic Regulation of BST-2 Expression Levels and the Effect on HIV-1 Pathogenesis
Abstract
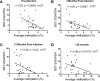
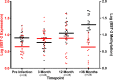
HIV-1 must overcome host antiviral restriction factors for efficient replication. We hypothesized that elevated levels of bone marrow stromal cell antigen 2 (BST-2), a potent host restriction factor that interferes with HIV-1 particle release in some human cells and is antagonized by the viral protein Vpu, may associate with viral control. Using cryopreserved samples, from HIV-1 seronegative and seropositive Black women, we measured in vitro expression levels of BST-2 mRNA using a real-time PCR assay and protein levels were validated by Western blotting. The expression level of BST-2 showed an association with viral control within two independent cohorts of Black HIV infected females (r=-0.53, p=0.015, [n =21]; and r=-0.62, p=0.0006, [n=28]). DNA methylation was identified as a mechanism regulating BST-2 levels, where increased BST-2 methylation results in lower expression levels and associates with worse HIV disease outcome. We further demonstrate the ability to regulate BST-2 levels using a DNA hypomethylation drug. Our results suggest BST-2 as a factor for potential therapeutic intervention against HIV and other diseases known to involve BST-2.
Keywords: BST-2; DNA methylation; HIV-1; epigenetic regulation; expression.
Copyright © 2021 Singh, Ramsuran, Naranbhai, Yende-Zuma, Garrett, Mlisana, Dong, Walker, Abdool Karim, Carrington and Ndung’u.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The handling Editor declared a shared affiliation, though no other collaboration, with one of the authors [MC].
Figures



References
-
- Sivro A, Su RC, Plummer FA, Ball TB. Interferon Responses in HIV Infection: From Protection to Disease. AIDS Rev (2014) 16:43–51. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

